Battery manufacture relies on the electrode materials being applied as a slurry, to form a film. The size and shape of particles within the slurry are critical to the production of the film and its final uniformity, via the viscosity of the slurry. In this application note, the Morphologi automated image analysis system is used to characterize efficiently the shape of particles in high and low viscosity electrode slurries.
Batteries are ubiquitous in modern life and our reliance on them has never been greater. Therefore, ensuring optimum battery performance through manufacturing control is of increasing significance. In previous application notes we have discussed the importance of controlling the size of particles used in the manufacture of battery materials [1] and the impact of carbon microstructure in graphite electrodes on battery performance [2].
Shape is also an important factor to consider and control, as irregular shaped particles not only reduce packing density, but they can lead to the formation of a high viscosity electrode slurry. In this third application note on batteries, written in cooperation with our partners from NETZSCH Analyzing & Testing, we consider the role of size and shape on the viscosity of the electrode slurry.
The typical structure of a battery electrode is given in Figure 1. The electrode is usually fabricated by applying a slurry of particles in suspension onto a metal foil.
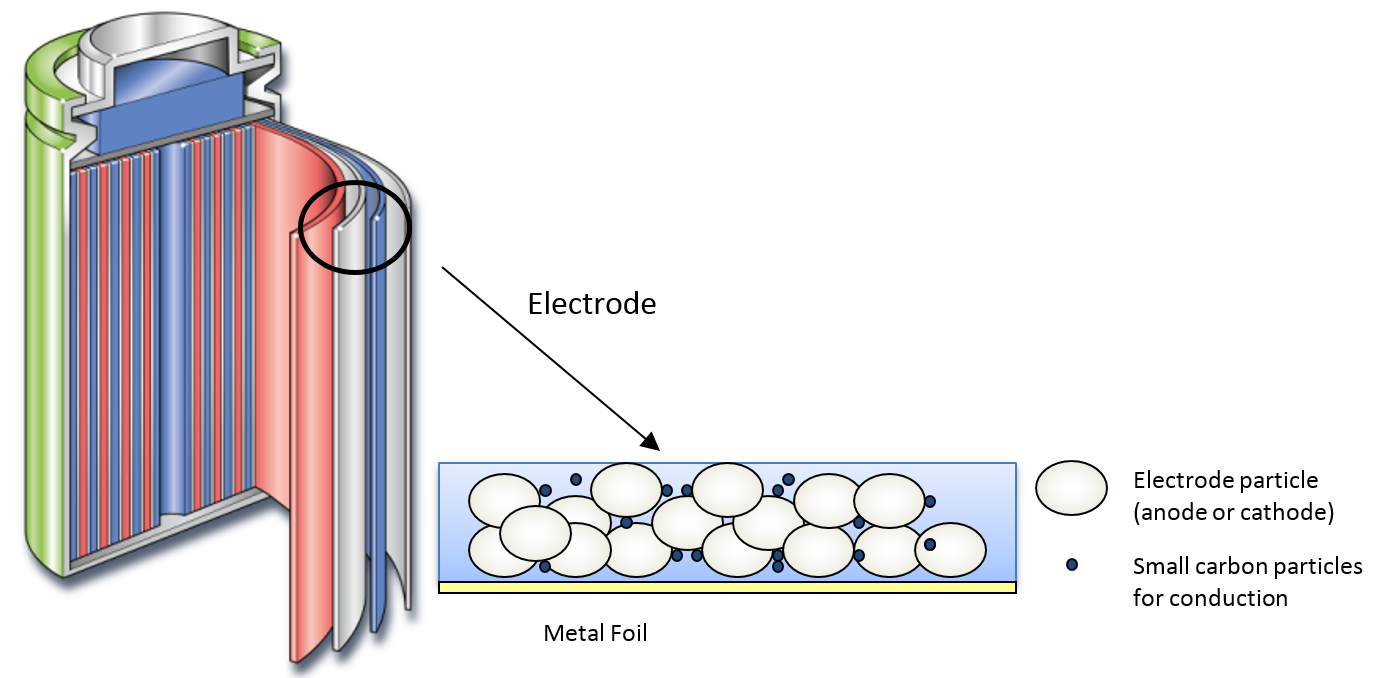
Figure 1 – typical structure of the Li-ion battery.
The slurry in question is composed of electrode particles (anode or cathode), small carbon particles to aid conduction, and binder material (composed of solvent and polymer) to hold the structure together. The concentration of particles in the slurry is high, representing between 20-40 % of the total by weight. Consequently, the particle properties have a significant impact on the physical properties of the resultant slurry.
The viscosity, dispersibility, concentration and compactability of the slurry are important parameters in determining how effective the slurry will be during application. A high viscosity slurry causes difficulties in the coating process, and poor dispersibility results in low film uniformity; the concentration and compactability of the slurry controls the film density. Uniformity of the coating thickness and the layer density are important to ensure control over the ion transfer rate and lifetime (recharge cycle time) of the battery, while controlling the layer thickness enables a smaller battery to be produced.
As illustrated in Figure 2, the presence of a significant proportion of irregularly shaped particles will lead to a higher viscosity slurry due to the increased impact of particle friction and interlocking but also due to the extra flow energy required for the fluid to circumvent the particles.
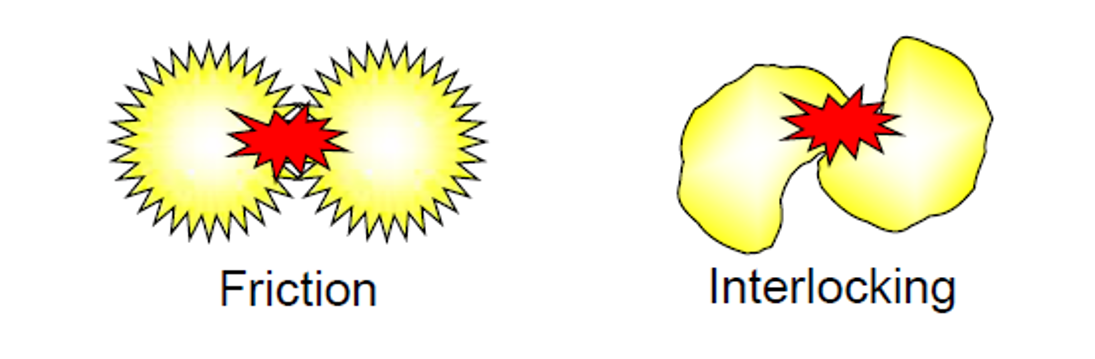
Figure 2 – irregularly shaped particles experience greater interlocking and friction, leading to a higher viscosity.
Particle shape also impacts on packing density since irregular particles pack less efficiently than spheres. Hence, fewer particles can be added to the liquid before the viscosity starts to increase, as illustrated in Figure 3. Further, a polydisperse sample will pack more efficiently than a monodisperse sample, at the same concentration, which will lower the viscosity. Smaller irregular particles, however, may increase viscosity due to their higher surface area, which will accentuate particle-particle and particle-liquid interactions. Therefore, it is important to be able to monitor and control the proportion of irregularly shaped particles and fine material within an electrode material sample to minimize the viscosity.
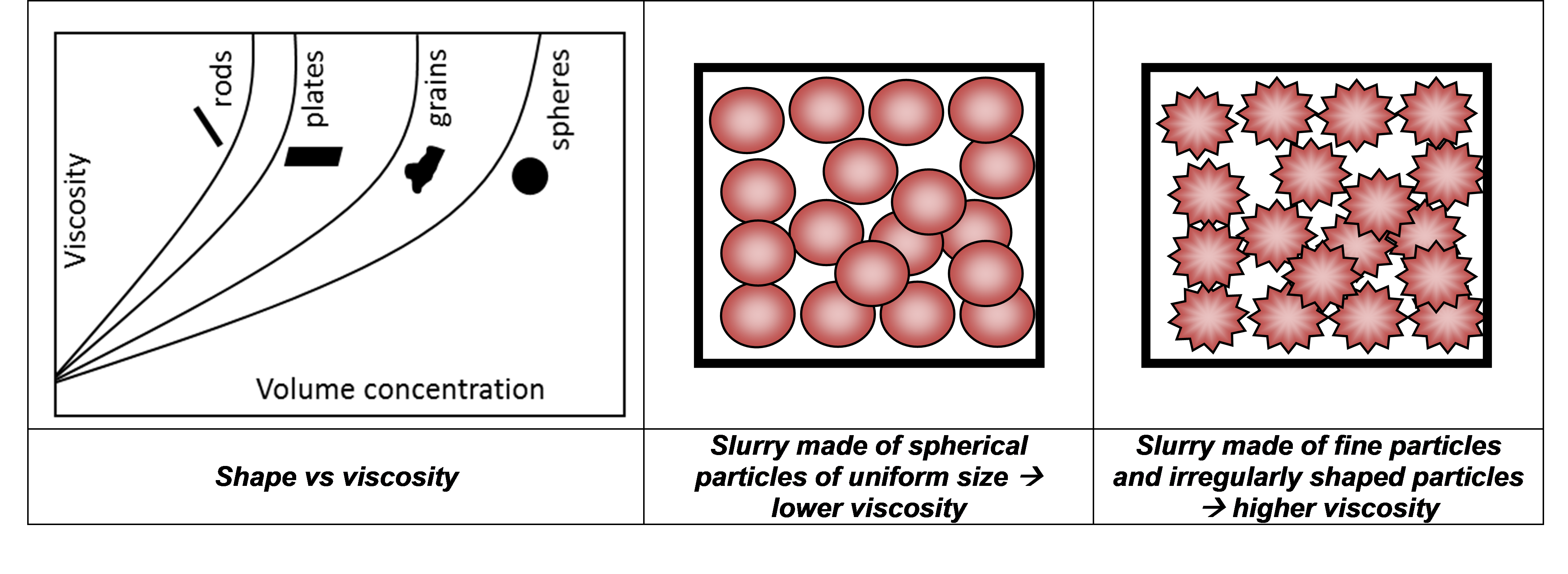
Figure 3 - the influence of particle shape on viscosity.
Login or create an account to read more about this case study.
Batteries are ubiquitous in modern life and our reliance on them has never been greater. Therefore, ensuring optimum battery performance through manufacturing control is of increasing significance. In previous application notes we have discussed the importance of controlling the size of particles used in the manufacture of battery materials [1] and the impact of carbon microstructure in graphite electrodes on battery performance [2].
Shape is also an important factor to consider and control, as irregular shaped particles not only reduce packing density, but they can lead to the formation of a high viscosity electrode slurry. In this third application note on batteries, written in cooperation with our partners from NETZSCH Analyzing & Testing, we consider the role of size and shape on the viscosity of the electrode slurry.
The typical structure of a battery electrode is given in Figure 1. The electrode is usually fabricated by applying a slurry of particles in suspension onto a metal foil.

Figure 1 – typical structure of the Li-ion battery.
The slurry in question is composed of electrode particles (anode or cathode), small carbon particles to aid conduction, and binder material (composed of solvent and polymer) to hold the structure together. The concentration of particles in the slurry is high, representing between 20-40 % of the total by weight. Consequently, the particle properties have a significant impact on the physical properties of the resultant slurry.
The viscosity, dispersibility, concentration and compactability of the slurry are important parameters in determining how effective the slurry will be during application. A high viscosity slurry causes difficulties in the coating process, and poor dispersibility results in low film uniformity; the concentration and compactability of the slurry controls the film density. Uniformity of the coating thickness and the layer density are important to ensure control over the ion transfer rate and lifetime (recharge cycle time) of the battery, while controlling the layer thickness enables a smaller battery to be produced.
As illustrated in Figure 2, the presence of a significant proportion of irregularly shaped particles will lead to a higher viscosity slurry due to the increased impact of particle friction and interlocking but also due to the extra flow energy required for the fluid to circumvent the particles.

Figure 2 – irregularly shaped particles experience greater interlocking and friction, leading to a higher viscosity.
Particle shape also impacts on packing density since irregular particles pack less efficiently than spheres. Hence, fewer particles can be added to the liquid before the viscosity starts to increase, as illustrated in Figure 3. Further, a polydisperse sample will pack more efficiently than a monodisperse sample, at the same concentration, which will lower the viscosity. Smaller irregular particles, however, may increase viscosity due to their higher surface area, which will accentuate particle-particle and particle-liquid interactions. Therefore, it is important to be able to monitor and control the proportion of irregularly shaped particles and fine material within an electrode material sample to minimize the viscosity.

Figure 3 - the influence of particle shape on viscosity.
In this study, two types of carbon material were investigated for use as a carbon electrode material: Carbon A, from natural sources, and synthetically produced Carbon B. Both materials were combined with the same binder (2.5% PVDF by weight in NMP) to form two slurries at a concentration of 22% by weight.
Viscosity measurements were made using a NETZSCH Kinexus rotational rheometer at shear rates ranging from 0.1 to 1000 s-1. Figure 4 shows that the addition of PVDF to NMP increases the viscosity by one order of magnitude (approximately 20 times) relative to NMP alone and the viscosity remains largely independent of shear rate (Newtonian behavior).
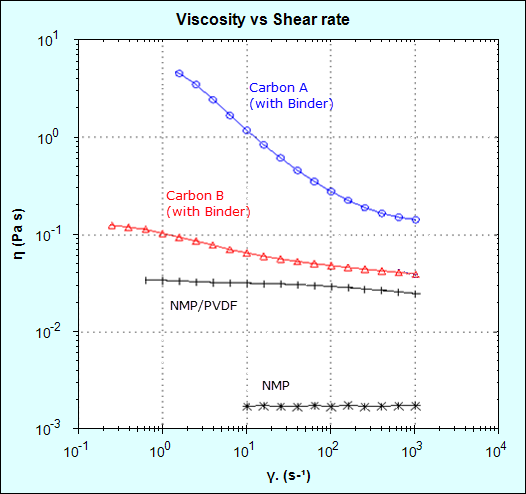
Figure 4 – slurry with Carbon A (naturally occurring) has a much higher viscosity than Carbon B (synthetically produced).
Adding carbon black further increased the viscosity and the resulting slurries both showed shear rate dependence (non-Newtonian behavior). The slurry made with Carbon A gave a much higher viscosity than Carbon B at low and high shear rates, which would likely increase resistance to sedimentation on standing (low shear process) and result in a thicker electrode film on coating (high shear process). The higher viscosity may also make the coating process more difficult to control, potentially leading to an uneven coating and variable layer density, which in turn results in a variable ion transfer rate and hence battery lifetime (and recharge cycle time).
In order to determine the cause of the differences in viscosity, both carbon powder samples were analyzed using the Malvern Panalytical Morphologi. The samples were dispersed using a low energy dispersion of 1 bar and over 70, 000 particles were automatically measured using the 10x objective.
As Figure 5 illustrates, it was found that the carbon material obtained from natural sources contained more fine material than the carbon sample generated synthetically.
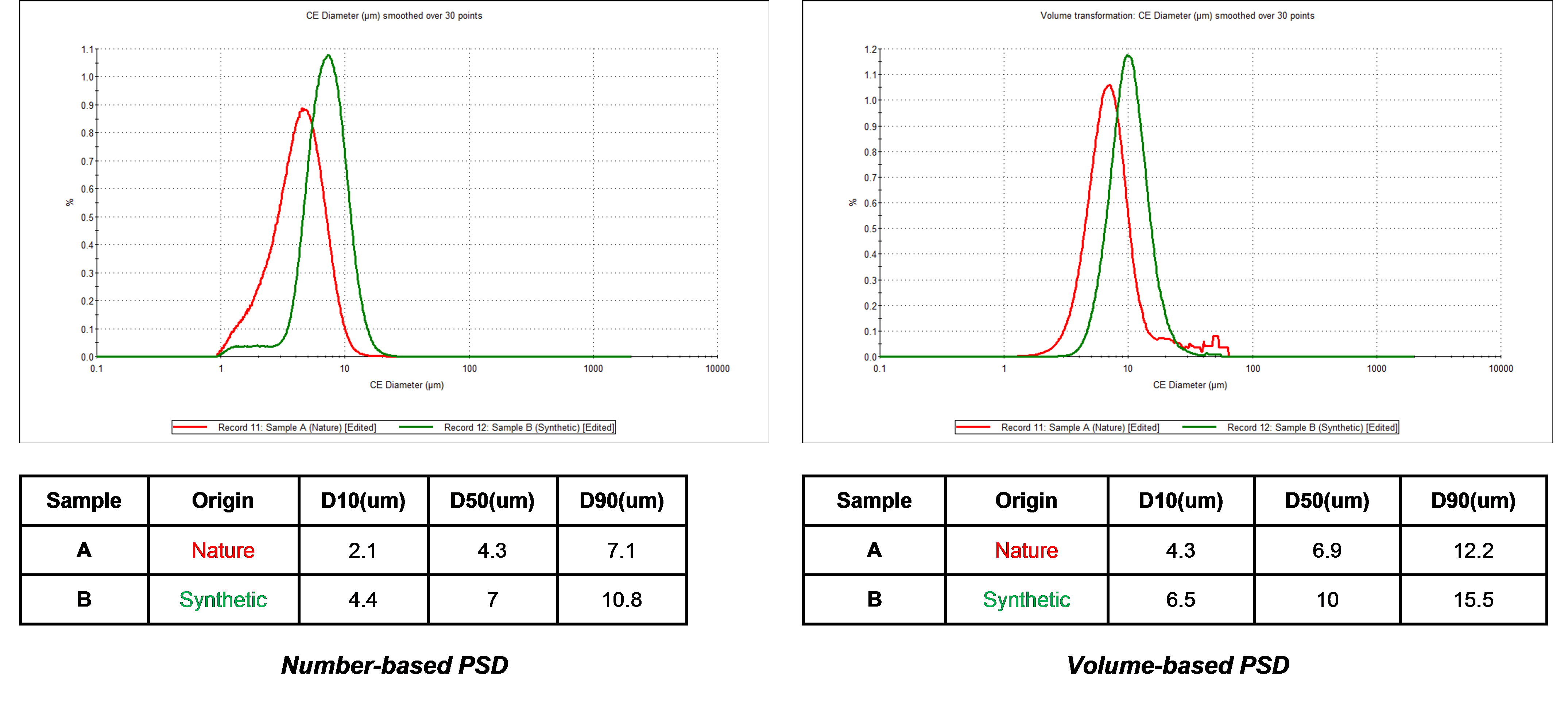
Figure 5 – size distributions for the naturally occurring carbon (red) and synthetically produced carbon (green).
Furthermore, it was found that although there was little difference in aspect ratio between the two carbon samples, comparing circularity revealed that Carbon B, the synthetic carbon material, has a higher circularity than that from natural sources, Carbon A, as shown in Figure 6. This is confirmed from the particle images shown in Figure 7.
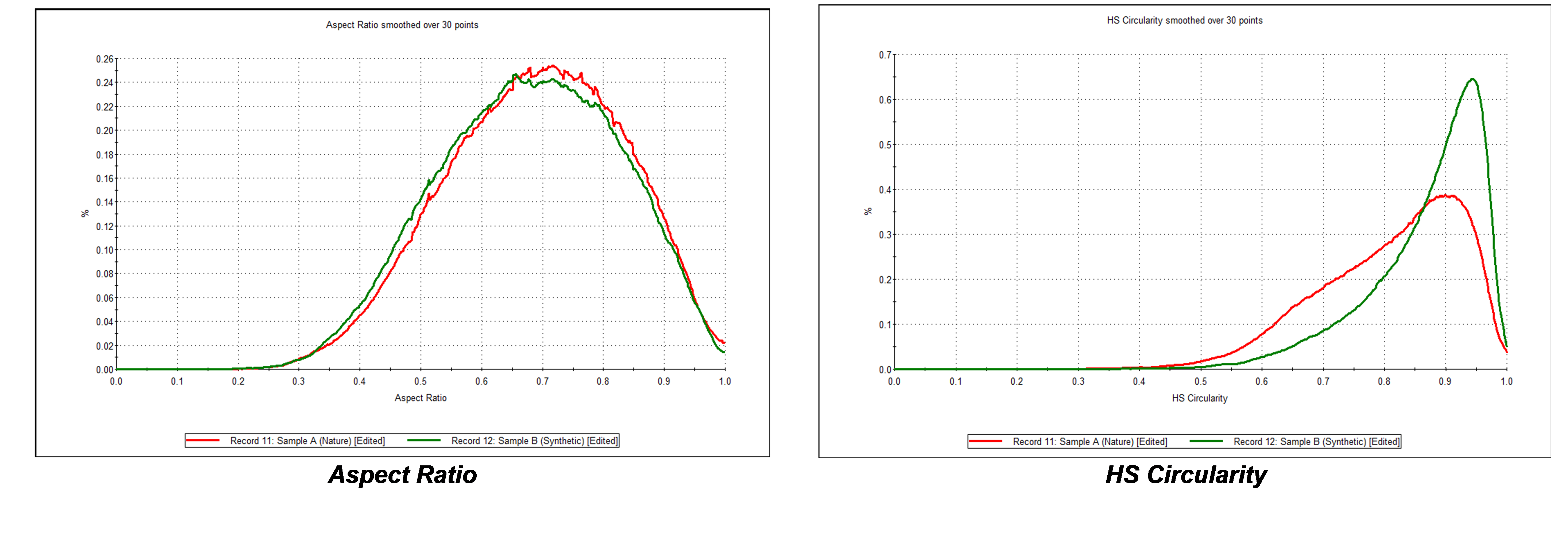
Figure 6 – the synthetically produced carbon (green) is more circular than the natural occurring carbon material (red) but there is little difference in aspect ratio.
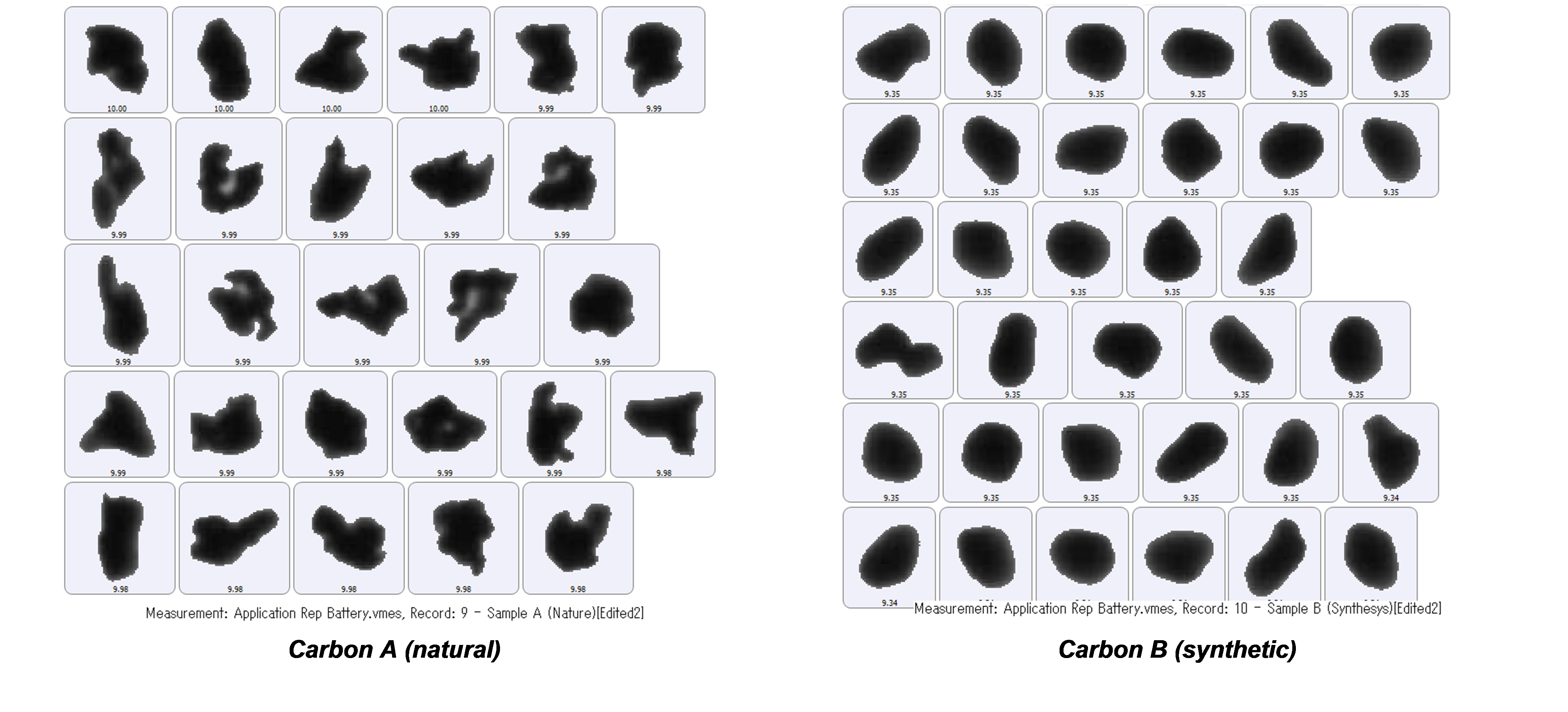
Figure 7 – particle images illustrating the differences observed in particle shape - the naturally occurring Carbon A has a much lower circularity than the synthetically produced Carbon B.
Two carbon-based electrode materials have demonstrated quite different viscosities when made into a slurry, resulting in differing application behaviors during the manufacture of batteries. Using the Malvern Panalytical Morphologi, we have been able to show that the natural-source carbon contains a higher proportion of fine material and irregular particles. Hence, when dispersed into a slurry the natural-source carbon will produce higher viscosities and lower packing fractions. A higher viscosity slurry reduces coating control during application to the electrode foil, which can result in an uneven coating of varying density. This impacts on battery performance, as the subsequent variability in ion-transfer rate leads to an unpredictable battery lifetime. Therefore, with the use of the Malvern Panalytical Morphologi automated image analyzer and the NETZSCH Kinexus rotational rheometer, slurry particle characteristics and slurry viscosity can be monitored to ensure that these factors are controlled.
[1] Application note, “Characterization of Battery Materials using Laser Diffraction Particle Size Analysis”.
[2] Application note, “Exploring the effect of carbon microstructure on lithium-ion battery performance”.