The zeta potential of protein covered latex can be measured by the Zetasizer Advance range. Measurements can show whether such binding is reversible or irreversible and whether the adsorption of surfactant prevents subsequent protein adsorption. Pluronic F68 adsorption to latex beads (200nm and 400nm) is observed by size and zeta potential.
Protein adsorption to surfaces has been widely studied because of its importance to medical and biotechnological applications. For materials implanted into the body (e.g. catheters, stents and even latex particles used for drug delivery) the issue of biocompatibility arises. Here it is desirable to prevent the adsorption of plasma, or interstitial fluid, proteins to the implant. In biotechnology applications, proteins are often located at surfaces where they carry out useful functions. Examples of interfacial activity range from emulsification, to particulate media-bearing enzymes, to biosensing transducers. Some biosensors require the attachment of an antibody to a surface in such a way that it retains its binding activity. Here one is attempting to nullify the denaturing effects of the substantial van der Waals force exerted on the protein by the solid surface, and also (and more difficult) to orientate the binding sites (paratopes) so that they face away from the surface. Advanced biosensors using planar surface transducers, such as BIAcore and IAsys, attach antibody to a thin (ca 200nm) layer of protein-permeable, reactive hydrogel bound to the surface.
In the area of in vitro diagnostic devices, colored sub-micron latex particles are commonly used. With these, for example, an antibody on the latex binds a protein analyte in the sample with which it is mixed, the latex then moves through a white membrane by capillary action and some of it binds to a line of antibody plotted on the membrane generating a colored line. The intensity of the line is an indicator of the concentration of the analyte. Similar tests can also be constructed for smaller molecules (haptens), which can only bind to one antibody. These versatile tests are used for measurements in urine (e.g. pregnancy and drugs of abuse testing) as well as in whole blood (e.g. cardiac troponin levels in plasma which can indicate a cardiac infarct).
Blood plasma is a complex mixture, with many of the protein components it contains likely to foul the antibody-coated surface of the latex. This application note demonstrates the utility of zeta potential measurements in establishing the minimum level of surfactant needed to coat a latex particle and how the measurement can indicate the absorption of protein onto a surfactant-coated latex.
Polystyrene lattices with a carboxylated surface were supplied by Duke Scientific, USA. These were 200 nm and 400 nm in diameter, with a CV on size of better than 10%. Preliminary experiments indicated that the measured zeta potentials were invariant for both lattices over the concentration range 0.00002% to 0.002%(w/v). They were dispersed to a final concentration of 0.0004% (w/v) in 10mM sodium tetraborate buffer, pH 8.6 containing different concentrations of Pluronic (F68) and Tetronic (T908) surfactants from BASF. Zeta potential measurements were made with a Malvern Panalytical Zetasizer Advance at 25 °C.
Bovine serum albumin and human fibrinogen were supplied by The Sigma Chemical Company, Poole, UK and were dissolved in 10mM borate, pH 8.6 buffer to a stock concentration of 20mg/ml and 10mg/ml respectively. The stocks were centrifuged at 25,000 x g for 30 minutes to remove any particulates. Protein-coated latex was made by adding protein at a final concentration of 0.2 mg/ml to a suspension of latex so that its final concentration was 0.0004%. The protein was left to adsorb for one hour at 22 °C before measuring the zeta potential. Tetronic coating was performed using T908 at a final concentration of 0.001% with the latex at 0.00044% (w/v). To assess protein adsorption 9 volumes of the latex in T908 solution were added to 1 volume of 10 mg/ml protein, mixed and measured within 20 to 30 minutes at 25 °C.
At the concentrations used with the latex, the count rate of the protein and surfactant solutions were too low to give rise to any consistent zeta potential measurements.
Pluronic and Tetronic surfactants have hydrophilic polyethylene oxide (PEO) chains with a hydrophobic polypropylene oxide (PPO) central chain; the pluronic has two PEO chains, one from each end of the PPO, while the Tetronic has four PEO chains, two from each end of the PPO chain. For F68, the PPO is a 30mer and the PEO (both chains are the same) is a 78mer; the T908 has a 21mer PPO and four 114mers of EO. The PPO moiety adsorbs onto the latex and the PEO groups extend from the surface as a random coil.
From the zeta potential data shown in Figure 1, it is evident that the two lattices had different zeta potentials, and so would have different surface concentrations of carboxylate groups. At pH 8.6, all the carboxylate is expected to be ionized. The relationship between zeta potential and surface charge density has not been sufficiently developed in theory for one to convert between the two. Zeta potential is a useful measure as it can be used to indicate the conditions under which a latex will flocculate or aggregate. It could also be used as a QC specification, particularly for those wanting to covalently couple protein to latex through activated carboxylate groups.
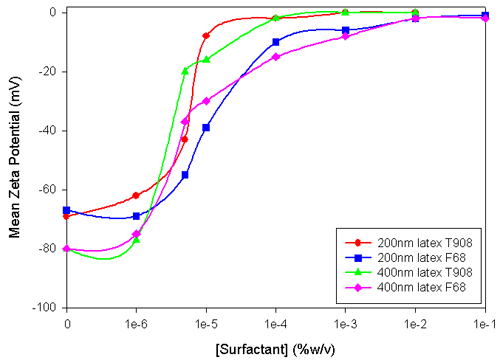
|
The zeta potential increased with the concentration of the surfactants, and when the latex was saturated with surfactant the zeta potential was less than 1mV. Such a low zeta potential would not impart electrostatic colloidal stability to these lattices, but they did not aggregate because of the steric properties of the hydrophilic PEO chains. The PEO chains extend further out from the surface than the plane of shear of solvent over the bare particle (the site of origin of the zeta potential). The position of the plane of shear is generally believed to be within 1nm of the particle surface. The 76 ethylene oxide units have a contour length of about 22nm (0.3nm/monomer), and when in a random coil they would have a diameter of around 5nm. Small angle neutron scattering measurements indicate that F68 adsorbs to form a layer of thickness of about 2nm at low electrolyte concentrations (C. Washington & S.M. King, ISIS facility annual report 1999-2000). The double-layer decay length in 10mM borate is about 2nm and therefore, it is reasonable to assume that the plane of shear of the surfactant-coated latex is well outside that of the bare particle. The zeta potential data indicates that the thickness of the adsorbed surfactant layer is greater than indicated by the SANS measurements. The zeta potential plateau occurs at a similar concentration of F68 to that measured by Kayes and Rawlings (Colloid & polymer Science, 257, 622-629 (1979)).
Preliminary work showed that mixtures of surfactant and protein gave rise to low levels of scattered light resulting in poor data with low repeatability.
Latex with an adsorbed film of protein showed a reduced zeta potential compared to the base latex (figure 2). Interestingly, the zeta potential of the protein-coated latex did not appear to depend upon the original zeta potential of the latex, this suggested that either the surface was fully covered with protein and this masked the carboxylate groups, or that the protein, like the surfactant, moved the plane of shear away from the latex surface. The protein concentrations used were more than enough to saturate the latex. Albumin and fibrinogen are quite different molecules, the former has a globular structure of diameter ca 10nm and a pI of 5, while the latter is a fibrous protein of length ca 50nm and a pI of 6.1. One simple explanation for the lower zeta potential of the fibrinogen-coated lattices could be that the rod-like protein moves the plane of shear further out than the albumin.
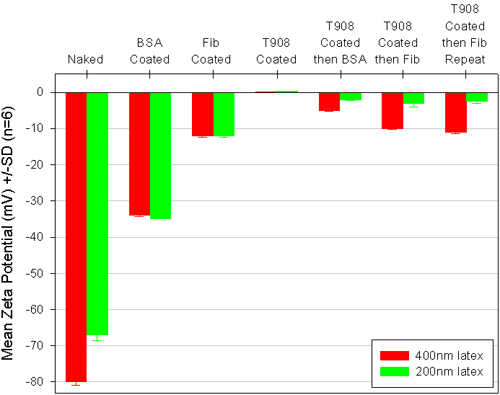
|
The idea behind using zeta potential as an indicator of protein adsorption on a Tetronic coated latex was that if protein did adsorb it should impart a surface charge to the latex at the ethylene oxide/water interface and so cause a change in the zeta potential. There is a substantial body of literature indicating that a polyethylene oxide coating greatly reduces protein adsorption and that the type of surfactants used here are not displaced by protein from the surface. Usually protein adsorption measurements are made by separating the latex from the protein solution and measuring the decrease in concentration of protein caused by adsorption to the latex. These "depletion" measurements need some care to achieve good data. Zeta potential measurements are relatively easy to make, and while not quantitative do provide a rapid and direct indication of protein adsorption to the latex.
When albumin was contacted with the T908 coated latex, the zeta potential decreased from +0.2mV to -3.5mV with the 400nm latex, and from +0.6mV to -1.3mV with the 200nm latex. Compared to the -33.5 mV for the albumin-coated lattices there appears to be far less adsorbed material on the T908 coated lattices. It is believed the PEO layer prevents binding of the albumin to the latex and the spherical nature of the protein limits the number of interactions it can have with the PEO layer.
With fibrinogen, the T908 coating had little effect on protein binding to the 400nm latex, but there was a clear suppression of adsorption to the 200nm latex. One explanation for these observations is that the rod-like fibrinogen is sufficiently flexible to make many low energy attractive interactions with the PEO layer but not flexible enough to match the higher curvature of the PEO on the 200nm diameter latex. A repeat experiment, of these surprising results, gave very similar findings.
The zeta potential of protein covered latex can quickly and reproducibly be measured by the Malvern Panalytical Zetasizer Advance range. The adsorption of surfactant can be determined as can the ability of the surfactant to prevent subsequent protein adsorption. The measurement can also show whether such binding is reversible (by dilution) or irreversible and may offer a rapid quality control measure on bare and finished latex used for diagnostic purposes.