Acceptance of and interest in the special properties of nanobubbles and other ultrafine bubbles is growing rapidly, and their formation and characteristics are the subject of an increasing amount of study, particularly in Europe and Japan. This white paper shows the degree to which the technique of Nanoparticle Tracking Analysis (NTA) can be used for the characterization of nanobubbles and other ultrafine bubbles.
NTA utilizes the properties of both light scattering and Brownian motion in order to obtain the particle size distribution of samples in liquid suspension. A laser beam is passed through the sample chamber, and the particles in suspension in the path of this beam scatter light in such a manner that they can easily be visualized via a 20x magnification microscope onto which is mounted a camera. The camera, which operates at approximately 30 frames per second (fps), captures a video file of the particles moving under Brownian motion within the field of view of approximately 100 μm x 80 μm x 10 μm (Figure 1).
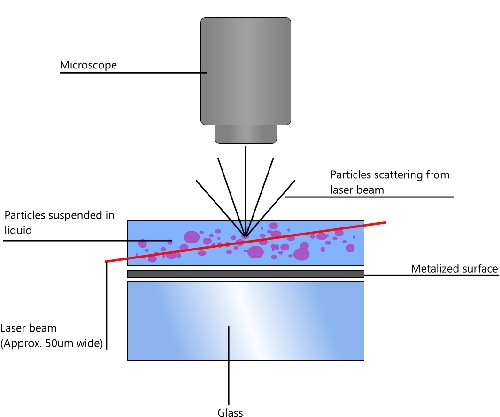
|
The movement of the particles is captured on a frame-by-frame basis. The proprietary NTA software simultaneously identifies and tracks the center of each of the observed particles, and determines the average distance moved by each particle in the x and y planes. This value allows the particle diffusion coefficient (Dt) to be determined from which, if the sample temperature T and solvent viscosity η are known, the sphere-equivalent hydrodynamic diameter, d, of the particles can be identified using the Stokes-Einstein equation (Equation 1).

|
NTA is not an ensemble technique interrogating a very large number of particles, but rather each particle is sized individually, irrespective of the others. An example of the size distribution profile generated by NTA is shown in Figure 2.
|
In addition, the particles' movement is measured within a fixed field of view (approximately 100 μm by 80 μm) illuminated by a beam approximately 10 μm in depth. These figures allow a scattering volume of the sample to be estimated; by measuring concentration of the particles within this field of view and extrapolating to a larger volume it is possible to achieve a concentration estimation in terms of particles per mL for any given size class or an overall total.
The generation, measurement, and applied technologies of extremely small ultrafine bubbles, so-called nano- and micro–bubbles, with diameter ranging from tens of nanometer to tens of micrometer, are evolving innovatively in recent years. Nanobubble technologies have already been implemented in actual applications such as facility cleaning, solar cell manufacturing process, plant growth, etc., and its application is considered to have the possibility to expand to wider range of fields, such as water treatment processing, environment, civil engineering, beverage, food, pharmaceutical, medical, cosmetic, plant cultivation, agriculture, fisheries, cleaning, decontamination, and also manufacturing of future functional materials. Therefore nano- and microbubble technology is expected to become one of the key players in major industries of the future. The existence of surface nanobubbles is becoming established following many different investigations from a number of groups. Far less common are reports of the existence of bulk nanobubbles. It has been argued that this is because they are considered less stable in bulk or that appropriate techniques for their investigation have not yet been developed.
Nanoparticle Tracking Analysis is proving to be particularly adept at the detection and analysis (size, size distribution, number concentration) of these relatively low concentration structures of extremely small size (compared to ‘conventional’ bubbles).
Seddon has recently and comprehensively reviewed the area of nanobubbles at surfaces and in bulk, and has considered the current understanding of their formation, stability, physicochemical properties and current and future applications (Seddon et al. (2012)). In principle, a nanobubble in the bulk should be less stable than one of the same volume at an interface. The bulk nanobubble has a larger gas/liquid interface to allow diffusion of gas out of the bubble. Also, the curvature of the bubble surface is greater, thus leading to a greater pressure differential across the interface for a bulk bubble of the same volume. Nonetheless, several groups have presented evidence for their existence and the most startling evidence for bulk nanobubbles is the recent work which reports small nitrogen, methane and argon bulk nanobubbles of radius 50 nm that are stable for up to 2 weeks. The bulk nanobubbles, which were produced by mechanical means that led to extreme supersaturation, were imaged from freeze-fracture replicas by SEM and were produced in such large quantities that the bulk density of the solution was substantially reduced.
It is noteworthy, however, that questions still remain over whether deeply sub-micron bubbles are what they are assumed to be. In a recent thought-provoking study Sedlak and Rak (2013) have shown that in solutions of low molar mass compounds and mixtures of liquids, large-scale inhomogeneities exist but which are not nanobubbles in all cases. Thus, despite the fact that in textbooks, undersaturated solutions of low molar mass compounds and mixtures of freely miscible liquids are considered as homogeneous at larger length scales exceeding appreciably dimensions of individual molecules, growing experimental evidence reveals that it is not the case. Large-scale structures with sizes on the order of 100 nm are present in degassed solutions and mixtures used in everyday life and research practice (e.g. atmospheric pressure), especially in aqueous systems. These mesoscale inhomogeneities are long-lived and their (relatively slow) formation kinetics can be monitored upon mixing the components using NTA. These results support experimental results obtained in earlier light scattering studies and, indeed, such results have been obtained (especially in 50:50 mixtures of water and ethanol) by the scientists responsible for the development of NTA (data not published).
Most of the work to date involving NTA analysis of nanobubbles has been carried out in Japan. Thus Takaya et al. (2011) described the formation of nanobubbles by water electrolysis and their analysis with NTA, while Kikuchi et al. (2011) investigated their stability and weight having determined their size distribution with NTA.
Uchida et al. (2011) used TEM observations of nanobubbles and their capture of impurities in wastewater. They generated a nanobubble solution by introducing pure O2 gas into the ultra-high purity water with a micro/nano bubble generator and used NTA to measure the resulting number concentration, estimated to be on the order of 107 cm-3 of solution under the same sample preparation conditions. Ushida also investigated the efficiency with which nanobubbles could replace detergents in the washing of laundry given it has been estimated that mechanical work has been found to account for 50% of the washing effect and nanobubbles can achieve the same mechanical action. A combination of nanobubbles and reduced detergency resulted in a 10% increase in washing efficiency (Ushida et al. (2011)). Ushidaet al. (2012) have recently investigated the drag reduction effect of nanobubble mixture flows through micro-orifices and capillaries in which the nanobubble-containing mixture was shown to contain 1.0 vol% nanobubbles by NTA. The results of studies using nanobubble mixtures for water and glycerol which were passed through several sizes of micro-orifices and capillaries suggested that the addition of nanobubbles to a liquid results in excellent drag reduction. Ushida also extended this work to include several types of nanobubble mixtures (nanobubble/water, nanobubble/surfactant and nanobubble/polymer) and discussed factors including slip wall, interfacial tension effect, electric interface phenomenon and elasticity (Ushida et al. (2013)).
Uehara and Yano (2011) have reported magnetized nanobubble water formed under a pulsed-magnetic field and Liu et al. (2013) have recently investigated the mechanism of nanobubbles’ physiological activity promotion with proton nuclear magnetic resonance (pNMR) relaxation time measurements. According to the experiment results, the number of nanobubbles had a positive correlation with the spin-spin relaxation time (T2) value of the water, which meant introducing nanobubbles could increase the mobility of water in bulk. These results suggested that the nanobubbles in water could influence the physical properties of water and that it could contribute to one of the explanations for the mechanism of nanobubble’s promotion effect on physiological activity of living organisms. The hydroponic experiment showed that the nanobubbles themselves could greatly promote the growth of barley and that nanobubble technology was possibly feasible to be used in hydroponic cultivation of vegetables as a new technology in agriculture applications. NTA was used to measure the bubble size diameters, a crucial parameter in understanding the effects they exhibited.
It is interesting to note that methods for the production and apparatus for the generation of nanobubbles, and in which NTA is used for analysis for supporting data, is currently the subject of recent patent activity (e.g. Ryu, 2012; Tsuji, 2012 and Tsuji et al. (2013); Lynn, 2013a and 2013b).
Numerous industrial applications of the use of nanobubbles are beginning to appear in the rapidly growing body of literature on the subject of nanobubbles. Those in which NTA is central to their analysis include studies on applications as diverse as petrochemicals and fuels, building materials and remediation of contaminated land sites and aquaculture. Ueda et al. (2013) described the use of water containing air bubbles with a diameter around 100 nm (nanobubbled water) on removal of radioactive carbon from granule conglomerate, asphalt and concrete contaminated sites in Fukushima, Japan. In a wide ranging study of the efficacy of water containing nanobubbles of air or oxygen gas as generated using a nanobubble aerator, Ebina et al. (2013) showed significant (compared to normal water) increases in growth (plant height, leaf length and fresh weight) of Brassica campestris grown using nanobubbled water; weight and length of DBA1/J mice free-fed nanobubbled water, as well as sweetfish and rainbow trout grown in nanobubbled water.
Nanodroplets that encapsulate a perfluoropentane (PFP) core will transition upon exposure to ultrasound pulses into gas microbubbles, which will rapidly expand and collapse resulting in disruption of cells similar to the histotripsy process but at a significantly lower acoustic pressure. Thus, in attempting to develop an image-guided, targeted ultrasound ablation technique by combining histotripsy with nanodroplets that can be selectively delivered to tumor cells, Vlaisavljevich et al. (2013) used NTA in the preparation of nanodroplets with an average diameter of 204 nm at 37 °C by self-assembly of an amphiphilic triblock copolymer around a PFP core, followed by cross-linkage of the polymer shell forming stable nanodroplets. Using a high speed camera to monitor microbubble generation, the peak negative pressure threshold needed to generate bubbles >50 μm in agarose phantoms containing nanodroplets was measured to be 10.8 MPa, which is significantly lower than the 28.8 MPa observed using ultrasound pulses alone. High speed images also showed that cavitation microbubbles produced from the nanodroplets displayed expansion and collapse similar to histotripsy alone at higher pressures. Nanodroplet-mediated histotripsy created consistent, well-defined fractionation of red blood cells in agarose tissue phantoms at 10 Hz pulse repetition frequency; similar to the lesions generated by histotripsy alone but at a significantly lower pressure. These results support their hypothesis and demonstrate the potential of using nanodroplet-mediated histotripsy for targeted cell ablation.
Finally, nanobubbles of air have been introduced into gas oil for energy saving and environmental load reduction of diesel engines. After the micro air-bubbles were separated from the nano air-bubbles in a mixing tank, diesel engine performance test with a common-rail injection system was tested. The results showed a 3% reduction in a brake specific fuel consumption (BSFC), 1% rise in charging efficiency and a slight reduction in the density of exhaust smoke (Nakatake et al. (2013)). Similarly, Oh et al. (2013) investigated the effect of hydrogen nanobubble addition on the combustion characteristics of a gasoline engine. Using NTA to demonstrate a mean diameter and concentration of hydrogen nanobubble in the gasoline blend of 149 nm and about 11x108 particles/mL, respectively, the results showed that the power of a gasoline engine with hydrogen nanobubble gasoline blend was improved by 4.0 % in comparison with conventional gasoline at an engine load of 40 %. Also, BSFC was improved, from 291.10 g/kWh for the conventional gasoline, to 269.48 g/kWh for the hydrogen nanobubble gasoline blend, at the engine load of 40%.
Seddon JRT, Lohse D, Ducker WA and Craig VSJ (2012) A Deliberation on Nanobubbles at Surfaces and in Bulk, ChemPhysChem 2012, 13, 2179 – 2187
Sedlak M and Rak D (2013) Large-Scale Inhomogeneities in Solutions of Low Molar Mass Compounds and Mixtures of Liquids: Supramolecular Structures or Nanobubbles?, J. Phys. Chem. B, Just Accepted Manuscript, DOI: 10.1021/jp4002093, Publication Date (Web): February 1, 2013
Takaya M., Kikuchi K, Oku T, Tanaka Y, Saihara Y and Ogumi Z (2011) Interface structure of oxygen nanobubble, Proc 61st Annual Meeting of the International Society of Electrochemistry, September 26th - October 1st, 2010, Nice, France
Kikuchi K, Ioka A, Oku T, Tanaka Y, Saihara Y and Ogumi Z (2011) Stability and weight of oxygen nanobubbles obtained with water electrolysis, Proc 61st Annual Meeting of the International Society of Electrochemistry, September 26th - October 1st, 2010, Nice, France
Tsuji, H; Tsuji Y, Oka T, Miyao H, Liauw D (2013) Extraction method using ultra fine bubbles and liquid extracts obtained thereof, United States Patent Application 20130045934, Publication Date: 02/21/2013
Uchida T, Oshita S, Ohmori M, Tsuno T, Soejima K, Shinozaki S, Take Y and Mitsuda K (2011) Transmission electron microscopic observations of nanobubbles and their capture of impurities in wastewater, Nanoscale Research Letters 6:295
Uehara K and Yano Y (2011) Magnetized Nanobubble Water Formed Under Pulsed-Magnetic Field, IEEE Transactions on Magnetics, Volume: 47 Issue:10, 2604 – 2607
Ushida A, Hasegawa T, Amaki K, Nakajima T, Takahashi N and Narumi T (2011), Investigation On Washing Effects For Nano-Bubble/Surfactant Mixtures In An Alternating Flow, Transactions of The Japan Society of Mechanical Engineers Series B, Vol. 77, No. 777 (2011), pp.1219-1228
Ushida A, Hasegawa T, Nakajima T, Uchiyama H, Narumi T (2012) Drag reduction effect of nanobubble mixture flows through micro-orifices and capillaries, Experimental Thermal and Fluid Science
Ushida A, Hasegawa T, Narumi T, Nakajima T (2013) Flow properties of nanobubble mixtures passing through micro-orifices, International Journal of Heat and Fluid Flow, Available online 17 February 2013, http://dx.doi.org/10.1016/j.ijheatfluidflow.2013.01.013
Uehara K and Yano Y (2011) Magnetized Nanobubble Water Formed Under Pulsed-Magnetic Field, IEEE Transactions on Magnetics, Volume: 47 Issue:10, 2604 – 2607
Liu S, Kawagoe Y, Makino Y, Oshita S (2013) Effects of nanobubbles on the physicochemical properties of water: The basis for peculiar properties of water containing nanobubbles, Chemical Engineering Science, Available online 11 February 2013 http://dx.doi.org/10.1016/j.ces.2013.02.004
Ryu, S-r (2012) Method and apparatus for generating nano-bubbles in liquid ,United States Patent Application 20120086137
Tsuji H, Tsuji Y, Oka T; Sugi S, Torii M, Miyao H, Nakayama Y, Torii T, Mori M (2012) Composition And Process For Production Thereof, United States Patent Application 20120128749
Tsuji, H; Tsuji Y, Oka T, Miyao H, Liauw D (2013) Extraction method using ultra fine bubbles and liquid extracts obtained thereof, United States Patent Application 20130045934, Publication Date: 02/21/2013
Lynn DW (2013a) Ozonated Liquid Dispensing Unit, US Patent 20,130,142,704, 2013
Lynn, DW (2013b) Ozonated Liquid Production And Distribution Systems, United States Patent Application 20130195725
Ueda Y, Tokuda Y, Fujimura S, Nihei N and Oka T (2013) Cesium Transfer from Granule Conglomerate, Asphalt, and Concrete Using Water Containing Nano-Sized Air Bubbles, ECS Trans. 2013 volume 50, issue 22, 1-6, doi: 10.1149/05022.0001ecst
Ebina K, Shi K, Hirao M, Hashimoto J, Kawato Y, et al. (2013) Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLoS ONE 8(6): e65339. doi:10.1371/journal.pone.0065339
Vlaisavljevich E, Durmaz YY, Maxwell A, ElSayed M, Xu Z (2013), Nanodroplet-Mediated Histotripsy for Image-guided Targeted Ultrasound Cell Ablation, Theranostics, 2013; 3(11):802-815. doi: 10.7150/thno.6717
Nakatake Y, Kisu S, Shigyo K, Eguchi T, Watanabe T (2013) Effect of nano air-bubbles mixed into gas oil on common-rail diesel engine, Energy, http://dx.doi.org/10.1016/j.energy.2013.06.065
Oh SH, Yoon SH, Song H, Guen Han J, Kim J-M (2013) Effect of hydrogen nanobubble addition on combustion characteristics of gasoline engine, International Journal of Hydrogen Energy, Available online 4 October 2013, http://dx.doi.org/10.1016/j.ijhydene.2013.09.063