This document summarizes sample measurements performed on the Zetasizer Nano series, the Zetasizer APS and the Zetasizer µV.
These measurements were designed to test the performance of the Zetasizer systems using careful sample preparation and measurement design.
All Zetasizer units provide a Z-average figure which is the intensity weighted mean hydrodynamic size of the ensemble collection of particles measured by dynamic light scattering (DLS). Many samples are polydisperse - a range of material sizes - and as such to achieve distribution measurements it is important that the system can monitor sizes beyond the range of the peak mode.
|
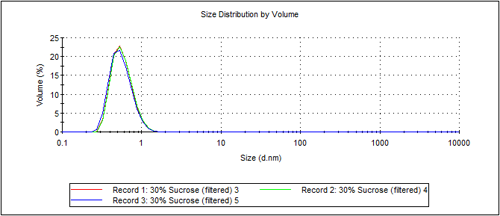
Figure 1 shows an overplot of 3 measurements of sucrose, at a concentration of 30%w/v. The peak had a mode of 0.6nm. To characterize this size, the system has a capability of measuring the size of the distribution down to 0.3nm
Figure 2 shows an overplot of three measurements of an 8.9 micron polystyrene latex, dispersed in 13%w/v sucrose. This dispersant is chosen to density match the latex to prevent sedimentation. This demonstrates the capability of the Zetasizer Nano S and ZS to measure particles with a size mode of 8.9 microns. To characterize this size, the system has a capability of measuring the size of the distribution up to 10.0 microns

|
All Zetasizer units provide a Z-average figure which is the intensity weighted mean hydrodynamic size of the ensemble collection of particles measured by dynamic light scattering (DLS). Many samples are polydisperse - a range of material sizes - and as such to achieve distribution measurements it is important that the system can monitor sizes beyond the range of the peak mode.
Figure 3 shows an overplot of 3 measurements of sucrose, at a concentration of 20%w/v. The peak had a mode of 0.6nm. To characterize this size, the system has a capability of measuring the size of the distribution down to 0.3nm.

|
Figure 4 shows an overplot of three measurements of a 3.0 micron polystyrene latex, dispersed in 13%w/v sucrose. This dispersant is chosen to density match the latex to prevent sedimentation. This demonstrates the capability of the Zetasizer Nano S and ZS to measure particles with a size mode of 3.0 microns. To characterize this size, the system has a capability of measuring the size of the distribution up to 5.0 microns

|
All Zetasizer units provide a Z-average figure which is the intensity weighted mean hydrodynamic size of the ensemble collection of particles measured by dynamic light scattering (DLS). Many samples are polydisperse - a range of material sizes - and as such to achieve distribution measurements it is important that the system can monitor sizes beyond the range of the peak mode.
Figure 5 shows an overplot of 3 repeat measurements of sucrose, at a concentration of 20%w/v. The peak had a mode of 0.3nm. To characterize this size, the system has a capability of measuring the size of the distribution down to 0.15nm

|
Figure 6 shows the measurement of a 0.5 micron radius polystyrene latex, dispersed in 13% w/v sucrose. This dispersant was chosen to density match the latex to prevent sedimentation. This demonstrates the capability of the Zetasizer APS to measure particles with a size mode of 0.5 microns. To characterize this size, the system has a capability of measuring the size of the distribution up to 1.0 micron.
|
All Zetasizer units provide a Z-average figure which is the intensity weighted mean hydrodynamic size of the ensemble collection of particles measured by dynamic light scattering (DLS). Many samples are polydisperse - a range of material sizes - and as such to achieve distribution measurements it is important that the system can monitor sizes beyond the range of the peak mode.
Figure 7 shows an overplot of 3 measurements of sucrose, at a concentration of 15%w/v. The peak had a mode of 0.3nm. To characterize this size, the system has a capability of measuring the size of the distribution down to 0.15nm.

|
Figure 8 shows an overplot of three measurements of a 0.5 micron radius polystyrene latex, dispersed in 13%w/v sucrose. This dispersant was chosen to density match the latex to prevent sedimentation. This demonstrates the capability of the Zetasizer µV to measure particles with a size mode of 0.5 microns. To characterize this size, the system has a capability of measuring the size of the distribution up to 1.0 micron.

|
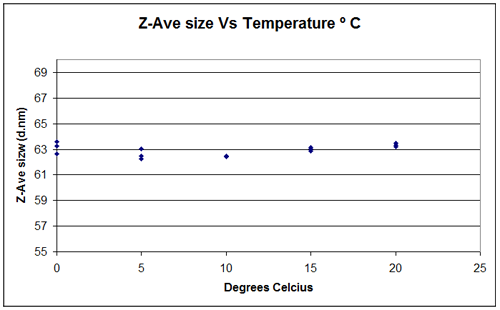
It is difficult to measure the temperature of a sample without affecting the results. In design and production, specialist equipment is used to measure the temperature at the extremes of performance, and also the rate of change of temperature. An alternative way of assessing temperature control and stability is to monitor the size of a known standard with which temperature deviation will be picked up by size variation. To test the Zetasizer a 60nm latex standard was measured three times in 10mM NaCl solution at a temperature of 20°C and the result recorded. This initial measurement was 63.43nm diameter with a SD of 0.14nm which is within the accepted tolerance limits. The temperature was then reduced in steps of 5°C down to 0°C with three measurements taken at each step. If the sample did not achieve the set temperature then you would expect to see variations in the measured size. The sizes measured at the different temperatures are shown in figure 9 and they indicate that the Zetasizer is capable of achieving set temperatures within +/-0.1°C.
Note: This relies on the viscosity being set correctly, and in the Zetasizer, for water, this is set automatically
|
The minimum concentration is specified using a 60nm latex. Figure 10 shows an overplot of 5 measurements of a 60nm latex, diluted in 10mM NaCl. The average size recorded was 63.7nm, with a standard deviation of 0.19nm.

|
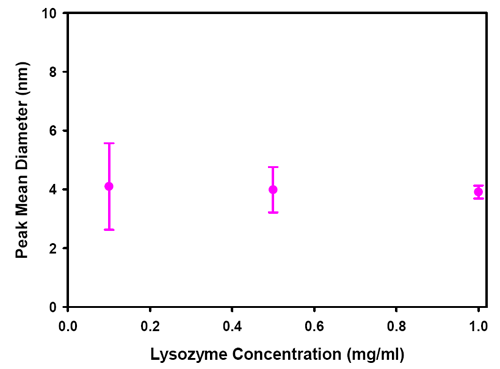
Figure 11 shows a plot of the size of peak 1 of measurements of lyzozyme as a function of concentration. Each point shows an error bar which is one standard deviation of a set of 5 repeat measurements. At 0.1mg/mL, the error in the measurement of size is larger than at higher concentrations but the mean value is in the expected size range for lysozyme.
|
Figure 12 shows an overplot of 3 measurements of a nominally 100nm silica at a concentration of 40%w/v, demonstrating the repeatability of measurement at this concentration.

|
The measurement capability of the Zetasizer Nano was tested using a solution of lysozyme. This molecule has a hydrodynamic diameter of 3.8nm when dissolved in a salt solution.
Figure 13 shows the mobility measurement of 3 aliquots of sample, each measurement repeated 7 times. The lysozyme was prepared at two concentrations, 5mg/mL and 20mg/mL dispersed in 100mM KCl with 10mM BisTris. The average result of each of the aliquots is 0.7 +/- 0.1 mobility units, or +9mV in terms of zeta potential. This demonstrates the repeatability of the result at this size, and is in agreement with the referenced published literature value plotted as the black line.

|
To measure the maximum particle size for zeta potential, a 97 micron polystyrene latex sample was used (figure 14). The sample was dispersed in 13%w/v sucrose to density match the latex. This was done to in order to do a number of measurements before the particles sedimented from the measurement volume, to show measurement repeatability.

|
The optical and electronic design of the Zetasizer Nano series limits the mobility of particles that can be measured to 3,460 mobility units (µm.cm/V.s). In practice, the highest mobility measured of a particle in an aqueous dispersion is about 10 mobility units. This capability then counts as no effective maximum. For specification purposes a value is declared of > +/- 20 µm.cm/V.s
The range of mobilities that can be measured by the Zetasizer, for samples in aqueous systems is equivalent to a zeta potential of +/- 44,300mV, and even higher for samples in non-aqueous dispersants. For samples with a mobility of 10µm.cm/V.s in an aqueous dispersant at 25ºC, the zeta potential calculated using the Smoluchowski limit is 128mV. This capability counts as no effective maximum. For specification purposes a value is declared of > +/- 500mV
Figure 15 shows 10 consecutive measurements of 30nm Ludox, pH 10 adjusted to a conductivity of 209mS/cm using sodium chloride. A 60 second pause was used between each measurement.

|
Figure 16 shows a plot of zeta potential as a function of concentration for an aqueous polyurethane dispersion with the addition of 5% triethylamine, demonstrating the ability to measure samples up to 40%w/v

|
The absolute molecular weight is measured using a Debye plot. This requires the measurement of the intensity of a scatterer with a known Rayleigh ratio, usually toluene, followed by the measurement of the scattering intensity of a number of known concentrations of a solution of the sample.
This requires that the system is sensitive enough to detect the excess scattering from the sample over the dispersant, and that the count rate is exceptionally stable.
Figure 17 shows a Debye plot using three concentrations of a 980 Dalton polystyrene standard, showing the capability of the Zetasizer Nano S and ZS to measure the molecular weight.

|
The absolute molecular weight is measured using a Debye plot. This requires the measurement of the intensity of a scatterer with a known Rayleigh ratio, usually toluene, followed by the measurement of the scattering intensity of a number of known concentrations of a solution of the sample.
This requires that the system is sensitive enough to detect the excess scattering from the sample over the dispersant, and that the count rate is exceptionally stable.
Figure 18 shows a Debye plot using three concentrations of a 9,800 Dalton polystyrene standard, showing the capability of the Zetasizer Nano S90 and ZS90 to measure the molecular weight.

|
The absolute molecular weight is measured using a Debye plot. This requires the measurement of the intensity of a scatterer with a known Rayleigh ratio, usually toluene, followed by the measurement of the scattering intensity of a number of known concentrations of a solution of the sample.
This requires that the system is sensitive enough to detect the excess scattering from the sample over the dispersant, and that the count rate is exceptionally stable.
Figure 19 shows a Debye plot using three concentrations of a 980 Dalton polystyrene standard, showing the capability of the Zetasizer µV to measure the molecular weight.

|
The molecular weight of protein solutions can be measured on the Zetasizer Nano and the Zetasizer µV using a Debye plot. This involves measurement of a number of known concentrations of a solution of molecules at the scattering angle of the system. The error in using a single angle for measurement for a globular protein, rather than using a multi angle system, can be calculated theoretically. The specification is then set at an acceptable level of error.
For the Zetasizer Nano S/ZS there is a maximum 5% error for molecules with an Rg of 27nm (figure 20). From known relationships this is equivalent to a globular protein with a molecular weight of 20 million Daltons.
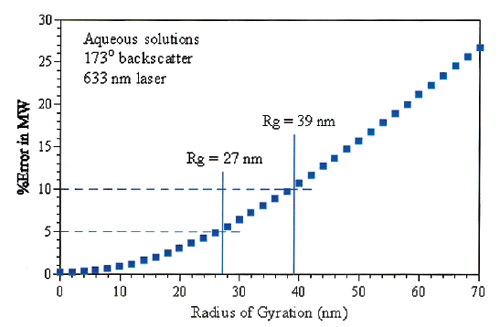
|
For the Zetasizer Nano S90/ZS90 and Zetasizer µV, the errors are much lower, hence the upper specification could be higher, however the specification is left at 20 million Daltons as this is sufficient to cover all practical samples.