The generation, measurement, and applied technologies of so-called nanobubbles or ultra-fine bubbles, with diameter ranging from tens to hundreds of nanometers, are evolving dramatically in recent years. The unique properties of nanobubbles makes them attractive for a number of applications. The Nanoparticle Tracking Analysis (NTA) technique is particularly adept at the detection and analysis (size, size distribution, number concentration) of these relatively low concentration structures of extremely small size (compared to ‘conventional’ bubbles). NTA offers a unique capability to characterize nanobubbles by directly visualizing nanoscale particles in suspension (10nm to 2000nm) with high-resolution, in real-time, and with minimal sample preparation.
The generation, measurement, and applied technologies of so-called nanobubbles or ultra-fine bubbles, with diameter ranging from tens to hundreds of nanometers, are evolving dramatically in recent years. The unique properties of nanobubbles makes them attractive for a number of applications such as facility cleaning, food disinfection, and water treatment, as well as possibilities in fields such as disinfecting food products, pharmaceutical delivery, decontamination, and manufacturing of functional materials. Preliminary market research conducted by the Fine Bubble Industries Association shows the size of the fine bubble business increasing from USD 20 million in 2010 to USD 4.3 billion in 2020.
The Nanoparticle Tracking Analysis (NTA) technique is particularly adept at the detection and analysis (size, size distribution, number concentration) of these relatively low concentration structures of extremely small size (compared to ‘conventional’ bubbles). NTA offers a unique capability to characterize nanobubbles by directly visualizing nanoscale particles in suspension (10 nm to 2000 nm) with high-resolution, in real-time, and with minimal sample preparation. The NanoSight models from Malvern Instruments pioneered this technique and continue to be the industry’s primary choice.
The existence of surface nanobubbles is becoming fairly well established following investigation from a number of groups. Following earlier skepticism about their actual existence, confirmation of bulk nanobubbles in solution and their characterization has more recently seen more publications.
Kaneo Chiba and Masayoshi Takahashi have shown that in the presence of electrolytes and with the correct physical stimulus, stable nanobubbles can be formed from conventional microbubbles [1]. The latter tend to coalesce to large buoyant bubbles which either float away or collapse under intense surface tension-derived pressure to the point that they vanish, as predicted by theory. However, the addition of salt (electrolytes) is thought to cause the formation of a counter-ion screen around the nanobubbles which effectively blocks the ability of gases within them to diffuse out (Figure 1).
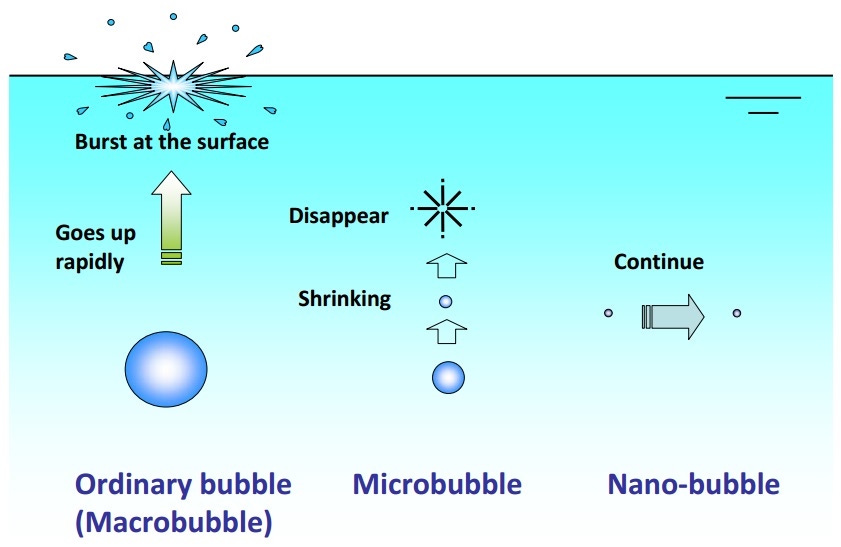
Figure 1. The formation of nanobubbles.
When measured without dilution, it is quite common to see nanobubble concentrations that are in the optimum concentration range for measurement with Malvern's NanoSight NTA instruments. This relatively low concentration means other traditional size analysis techniques like Dynamic Light Scattering (DLS) may not have enough signal to make a reliable size measurement.
The following example shows a nanobubble sample after being recirculated through the generator for a period of time. This was compared to the starting ‘Production Water” and against normal tap water. All samples showed some levels of particles present but the sample recirculated through the nanobubble generator showed total particle concentrations approximately five times higher than either of the other samples tested.
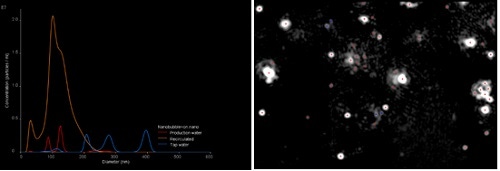
Figure 2. Comparison of NTA results for water recirculated through a nanobubble generator to tap water and production water and an image from the NTA analysis.
Zhu et al [2] investigated the efficacy of aqueous solutions containing nanobubbles for the removal of model contaminants, Bovine Serum Albumin and Lyzosome, from surfaces and prevention of fouling by the same proteins. Before they proceeded with the cleaning experiments, they used a NanoSight NS300 to confirm the formation of nanobubbles by electrolysis in different solutions:
| Solution | Description | Concentration (x 106particles/mL) |
| ec-H2O sub 450 nm | Electrolyzed purified water with 2.1 mM NaCl, 450 nm filtered | 750 ± 50 |
| NaCl(aq) sub 450 nm | Purified water with 2.1 mM NaCl, 450 nm filtered | 28 ± 2 |
| H2O sub 450 nm | Purified water, 450 nm filtered | 3 ± 1 |
| ec-H2O sub 20 nm | Electrolyzed purified water with 2.1 mM NaCl, 20 nm filtered | 3 ± 1
|
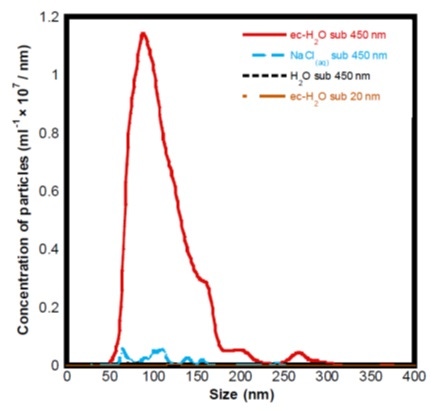
Figure 3. Histogram showing the particle size distributions and concentrations of different sample preparations obtained using the NanoSight NS300.
After concluding that nanobubbles were best formed in electrolyzed water with 2.1 mM NaCl and passed through a 450 nm filter, they further used NanoSight to determine the stability of these particles over time:
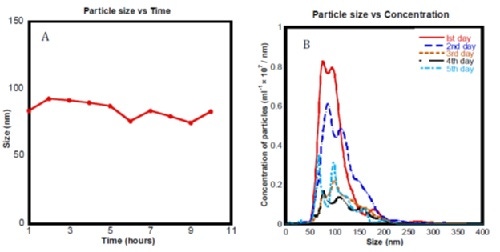
Figure 4. (a) Mean bubble size distribution for ec-H2O sub 450nm measured using NTA over 10 hours. (b) Histogram showing particle concentration measured using the NanoSight samples of ‘ec-H2O sub 450nm’ over 5 days.
Seung Hoon Oh et al. measured the long-term stability of hydrogen nanobubbles (HNB) in gasoline [3]. They are investigating the introduction of hydrogen to improve engine efficiency and emission performance. The generated the HNB-gasoline blend with a nanobubble generator through gas-liquid dispersion. Once nanobubbles were formed, they measured their stability with NanoSight for 121 days at a constant temperature of 25 C. After initial formation, the size and concentration were 159 ± 32 nm and 11.3 ± 2.8 x108 particles/mL, respectively. They saw minor variability but no significant trends in size and concentrations after 121 days.
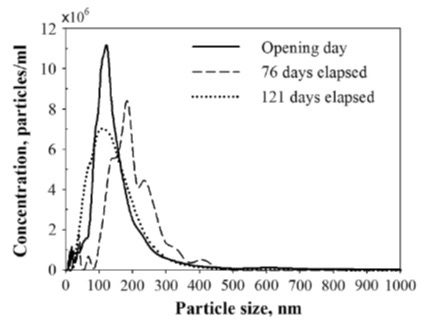
Figure 5. Size distribution of hydrogen nanobubbles versus time.
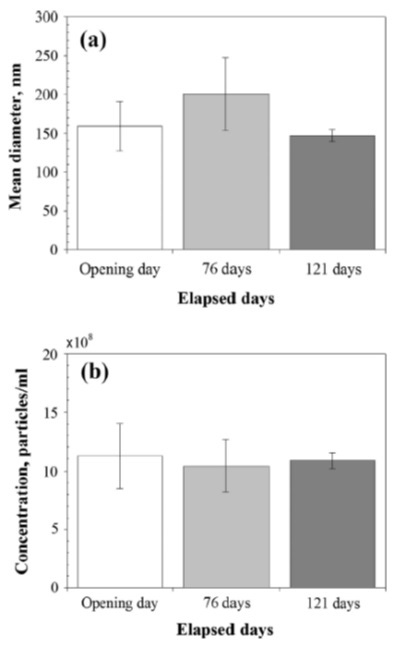
Figure 6. (a) Mean diameter and (b) concentration of hydrogen nanobubbles versus time
[1] Takahashi, M., “Zeta Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas-Water Interface.” J. Phys. Chem. B 109 21858–64, (2005)
[2] Zhu, J., et al. "Cleaning with Bulk Nanobubbles." Langmuir: the ACS journal of surfaces and colloids (2016).
[3] Oh, Seung Hoon, Jung Guen Han, and Jong-Min Kim. "Long-term stability of hydrogen nanobubble fuel." Fuel 158 (2015): 399-404.a