Titanium Dioxide is used in a wide range of industries to enhance colour and absorb UV light. Its particle size affects the opacity, color and gloss achieved when using the pigment, along with processing parameters such as viscosity and stability.
Please login or register for free to read more.
Titanium dioxide is used in a wide range of industries to enhance color and to absorb UV light, for example:
The whiteness and opacity is due to the high refractive index of titanium dioxide. This means that titanium dioxide is a very efficient scatter of light, making coatings appear bright white and effectively hiding anything underneath. Of its two main crystal structures: anatase and rutile; rutile is more commonly used as a pigment due to its higher refractive index, and therefore higher scattering efficiency.
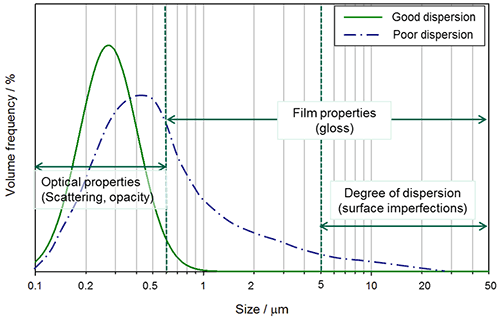
The size of pigment particles affects the opacity, color, gloss, viscosity and sedimentation rate of a suspension. The most important factor for titanium dioxide is the effect of particle size on scattering efficiency, as this determines opacity. Scattering efficiency is also dependent on wavelength: smaller particles will scatter more blue light whereas larger particles will scatter more red light. Hence particle size will also affect undertone. The optimum particle size for scattering white light is around 250nm. Particles larger than 0.5μm will affect the film properties such as gloss, and larger agglomerates in poor dispersions will cause surface imperfections, Figure 1[after 1].
|
The wide dynamic range of the Mastersizer, from 0.01μm to 3500μm, is ideally suited to following the milling process of pigments such as titanium dioxide. The enhanced submicron performance of Mastersizer instruments will also aid in the accurate characterization of the primary particles of titanium dioxide.
To measure the primary particle size accurately we must make sure that the material is fully dispersed. As particle size decreases the forces of adhesion between particles increase. A dry powder of titanium dioxide with a primary particle size of less than 500nm is, therefore, one of the more difficult materials to disperse. The Mastersizer has a range of dispersion units which can be used to disperse samples in liquids and as dry powders. The most appropriate method of dispersion will depend on the form of the sample and its particle size. Dry dispersion is an efficient way of measuring larger quantities of coarser particles, whereas wet dispersion offers more options for achieving a stable dispersion of very fine particles.
|
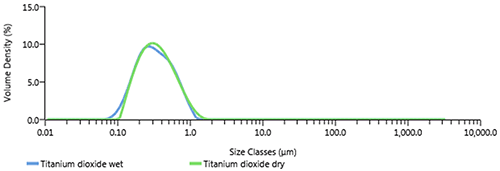
Dispersing titanium dioxide in water requires dispersing agents (such as sodium hexametaphosphate) and in some cases control of the pH of the dispersion [2], depending on the surface treatment of the material. In dry dispersion, a venturi driven by compressed air is used to disperse agglomerates. As titanium dioxide is a robust, agglomerated material the Aero S high energy venturi [3] needs to be used to disperse the sample. Figure 2 shows the particle size distribution for a titanium dioxide sample measured in water with added sodium hexametaphosphate using the Hydro EV liquid dispersion unit (after ultrasound), and dry dispersion using the Aero S high energy venturi at 4bar. These results show good agreement between liquid and dry dispersion which verifies that that the sample is fully dispersed in both states.
|
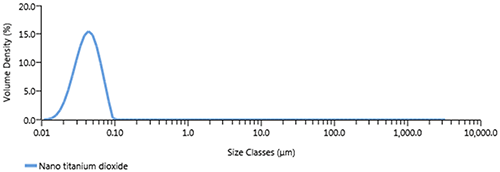
Titanium dioxide is used in sunscreens for its UV absorbing properties. In some products very fine particle sizes, sub 100nm, are used to produce products that appear clear when applied. These extremely fine particles are very difficult to re-disperse once dried so are generally produced as suspensions. Particles of this size must therefore be measured in wet dispersion. Figure 3 shows the particle size distribution for a sub 100nm titanium dioxide sample measured in wet dispersion using the Hydro EV.
Mie theory is used to interpret the angular scattering data measured by the Mastersizer to produce a particle size distribution. To use Mie theory we must input the optical properties of the system: the refractive index of the dispersant, the refractive index of the particles and the imaginary refractive index, or absorption, of the particles. These properties enable the scattering produced by fine particles to be modeled correctly. The Mastersizer uses two light sources, a primary red light source at 633nm and a secondary blue light source at 470nm. The scattering of titanium dioxide is highly wavelength dependent and therefore to characterize the material accurately by laser diffraction, separate optical properties for the red and blue light source may be required. Table 1 shows the refractive indices in both red and blue light for rutile and anatase titanium dioxide. Mie theory also requires the imaginary component of the refractive index, or absorption. Few references to particulate absorption are available although there are methods which can be used to determine the absorption value for a sample [5]. A low absorption of visible light is assumed for titanium dioxide as its hiding power is due to scattering rather than absorption, typically 0.01 is used for crystalline milled materials.
Structure | Refractive index (red) | Refractive index (blue) |
|---|---|---|
Rutile | 2.68 | 2.88 |
Anatase | 2.51 | 2.66 |
Laser diffraction is a widely used technique for the analysis of titanium dioxides due to its wide dynamic range, sub-micron performance and flexible options for both wet and dry sample dispersion.