Zeta potential has long been used as a useful parameter and indicator to study stability and other properties of particulate suspensions. For aqueous suspensions, mature theories and many research reports exist in the literature. However, even though there are many examples of applications for stability studies of non-aqueous suspensions, such as liquid inks, carbon black suspensions, liquid fuels, the correct measurement of the zeta potential of particles in non-aqueous media is not easy. Wrong parameter settings, limitations of instrumentation, and misinterpretation often leads to incorrect conclusions. The key to obtaining correct and useful information from zeta potential measurement in non-aqueous suspension is to choose a suitable instrument and accessory designed for these measurements, and to use the correct parameter settings.
The most popular method to measure zeta potential is electrophoretic light scattering, in which particles are move under an external electric field and their mobility is determined from the Doppler shift of scattered light; zeta potential being subsequently calculated using a proper theoretical model. Even though there are many commercial instruments that can be used to measure zeta potential, not many can be used to measure zeta potential in non-aqueous media.
The main reason is due to the low dielectric constants associated with non-aqueous systems resulting in very low velocity, hence low mobility, generated by the particle electrophoresis. Therefore, an instrument has to be able to detect mobilities orders of magnitude smaller than those usually encountered in aqueous dispersants.
Phase analysis light scattering (PALS) is a technique that has extremely high sensitivity for the detection of very small particle velocities and is the best technique currently available for measuring electrophoretic motion in non-aqueous suspensions. PALS measures the phase change of scattered light with respect to the incident light from which the electrophoretic mobilities, as low as 10-12 m2 /V.sec, can be determined.
Figure 1 shows an electrophoretic mobility distribution of carbon black in Isopar G with a mean mobility of 9.8 x 10-10 m2 /V/s measured using a Zetasizer Nano instrument using patented M3-PALS technology. The mean electrophoretic mobility value was determined from a PALS analysis and the distribution was obtained from a Fourier transformation of Doppler-shifted scattered light.
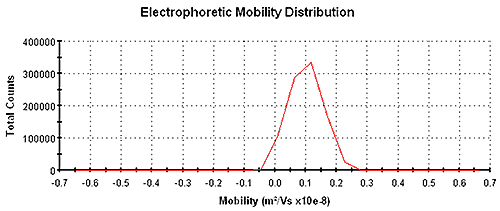
|
Two types of electrophoretic mobility measurement sample cells are commonly used: the capillary cell and a cell with closely spaced parallel electrodes, sometimes called a dip cell.
The capillary cell contains electrodes at both ends of a closed tube, and figure 2(a) shows the unique disposable one used in the Zetasizer Nano instrument. The advantage of this type of cell is that the measurement position is distant from the electrodes avoiding any influence from electrolysis and bubbles when measuring high conductivity samples. This type of cell is excellent for aqueous applications.

|
However, when very high electric field strengths are required to measure particles dispersed in low dielectric constant media such as non-aqueous solvents, it is of great advantage to use a dip cell configuration. This cell has two electrodes that are positioned closely together (figure 2(b)). Because the distance between the two electrodes in the dip-in cell is more than 10 times smaller than that in a capillary cell, the same voltage applied will produce a much stronger electric field. This avoids having to apply fields of thousands of volts to get the same mobility. In non-aqueous liquids, bubbles from electrolysis and Joule heating effects are less likely to occur as the conductivity will be far lower that for aqueous dispersants.

|
In order to obtain a reliable electrophoretic mobility result, there must be a measurable difference in frequency or phase between the incident light and scattered light. However, if the applied field is too high, adverse effects such as heating, turbulence, secondary field effects, non-homogenous fields, etc can occur. Often the reason for non-repeatability is too high an applied field. Therefore, the setting of the applied voltage should start at a low value, such as 5V or 10V, to produce a minimum of 1 to1.5 Hz in frequency shift. If the shift produced is too small or repeatable results cannot be obtained, then the field can be increased until satisfactory results are achieved. Repeat measurements will show whether the voltage is in the correct range to provide repeatable results. If samples in different dispersants are being measured, a thorough cleaning of the sample cell and electrodes is needed to avoid cross contamination.
Carbon black nanopowders are used in many industrial fields, including ink, paint, and coatings, asphalt sealants and decorative concrete colorants, textiles and conductive/resistive applications. In both dry powder and wet suspension, carbon black particles exist as aggregates due to their method of production and surface morphology. Even though many carbon black applications are in aqueous suspensions, there are several in which the carbon black has to be dispersed in non-aqueous media. There has been much effort spent, mostly based on empirical formulation, in finding the best medium to disperse carbon black particles to minimize the presence of aggregates. For different dispersion media, besides the molecular structural differences, one parameter that relates the charge transfer capability, and therefore the surface charge of dispersed particles, is the dielectric constant. A systematic way to find the best medium for the dispersion of carbon black particles is to measure its zeta potential, as well as its particle size in media of various dielectric constants.
Measurements of a carbon black nanopowder dispersed in a range of organic solvents were performed on Zetasizer Nano ZS in conjunction with a universal dip cell accessory. The solvents selected had dielectric constants ranging from 2 to 38 respectively. Small amounts of the powder were added to each solvent and sonicated for 3 minutes to aid dispersion prior to zeta potential measurement. In addition, particle size measurements were made using the dynamic light scattering capability of the Zetasizer Nano ZS.
Table 1 summarizes the electrophoretic mobilities, zeta potentials and particle sizes obtained from the carbon black powder dispersed in media with different dielectric constants. The electrophoretic mobilities were converted into zeta potentials using Huckel's approximation. The sizes reported are the z-average diameters, which is the intensity-weighted mean diameter as defined in ISO22412. The z-average size is very sensitive to the presence of aggregates. The data shown in table one is graphically represented in figure 3. Clearly, when the dielectric constant of the medium is lower than 5, large aggregates are present due to the very low zeta potential (<10mV). In liquids of dielectric constant greater than 7, particles have high zeta potentials ranging from -125 mV to -35 mV with z-average diameters around 150 nm and hence are very stable.
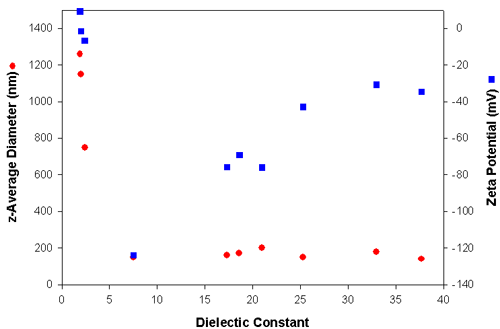
|
| Medium | Dielectric Constant | z-Average Diameter (nm) | Electrophoretic Mobility (see printout) | Zeta Potential (mV) |
|---|---|---|---|---|
| Heptane | 1.9 | 1260 | 0.027 | 9.2 |
| Cyclohexane | 2.0 | 1150 | -0.0038 | -1.5 |
| Toluene | 2.4 | 750 | -0.017 | -6.6 |
| Tetrahydrofuran | 7.5 | 150 | -1.2 | -124 |
| Butan-2-ol | 17.3 | 160 | -0.23 | -75.8 |
| Methylethylketone | 18.6 | 170 | -1.9 | -69.2 |
| Acetone | 21.0 | 200 | -2.9 | -76.0 |
| Ethanol | 25.3 | 150 | -0.57 | -42.8 |
| Methanol | 33.0 | 180 | -1.1 | -30.8 |
| 2-aminoethanol | 37.7 | 140 | -0.045 | -34.7 |
Successful zeta potential measurements of materials dispersed in non-aqueous solvents can be made on an appropriate instrument such as the Zetasizer Nano ZS in conjunction with a dip cell. The results obtained indicate that in this case, optimizing the dispersion in non-aqueous media is related to the dielectric constant of the solvent. Stable suspensions with small particle sizes were achievable in solvents whose dielectric constant was greater than 7. These experiments also demonstrate that the stability of carbon black particles in non-aqueous media is closely related to the zeta potential value obtained. Therefore these zeta potential measurements can be used to study and predict the dispersion stability of carbon black samples.