The physical stability of protein formulations is paramount in protein therapeutics. In particular, the presence of aggregates formed during manufacture and/or storage of protein formulations have been shown to induce adverse reactions.
Thus, characterization and early detection of aggregates is desirable during formulation development as well as after various storage/delivery induced stresses, such as freezing and thawing, agitation and needle administration.
This application note describes the use of dual angle dynamic light scattering (DLS) measurements for the enhanced detection of protein aggregation. The method takes advantage of the non-invasive character of DLS to ensure that the protein solution properties are not altered during analysis.
DLS uses the intensity fluctuations of scattered light to measure the size and size distribution of proteins in solution. These intensity fluctuations are induced by the movement of proteins due to collisions with the solvent molecules, also known as Brownian motion. Specifically, light scattered by molecules undergoing Brownian motion interferes either constructively or destructively depending on the relative positions of the protein molecules in the scattering volume. The rate of the detected intensity fluctuations is used to calculate a diffusion coefficient, which in turn is converted into a hydrodynamic size for the protein using the Stokes Einstein equation.
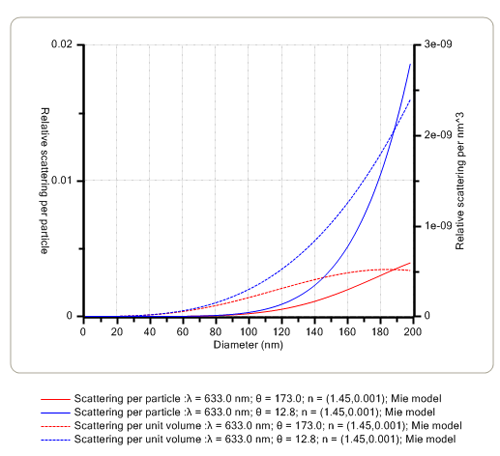
The angular dependence of the intensity of scattered light is affected mainly by the size of the proteins in solution. When the size is very small compared with the wavelength (λ) of the laser light, the scattered intensity is the same at all detection angles and hence, analysis at any angle will yield the same result. However, as the size of the protein or aggregate increases to be greater than ≈λ/10 and approaches the wavelength of the laser, the scattered intensity starts to increase in the forward direction. Thus, measurements in both the backward and forward scattering directions can be used to monitor the onset of protein aggregation. This is exemplified in Figure 1 below for two specific backward (173.0 degrees) and forward (12.8 degrees) scattering angles.
|
Dual angle DLS measurements were performed with the Zetasizer Nano ZS. The two detection angles used were 173.0 degrees and 12.8 degrees for backward and forward scattering measurements, respectively. Lysozyme and bovine serum albumin (BSA) were chosen to test the sensitivity to the presence of oligomers and aggregates. A volume of 1ml of the protein solution under investigation was placed in a glass cuvette and measurements were taken at 25°C. Aggregation of the BSA protein solution was induced by raising the temperature of the sample to 56°C. An aggregation index (AI) was calculated for each of the protein solutions measured. The aggregation index is based on the mean z-average size measured for the two angles of scattering according to the equation:
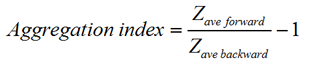
|
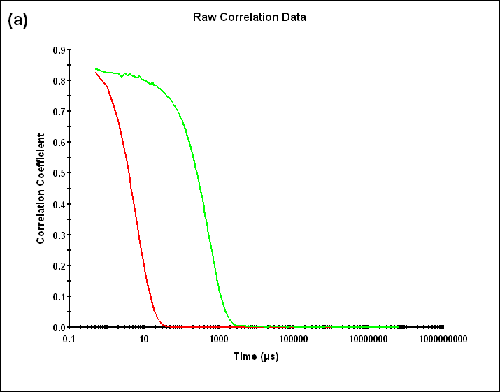
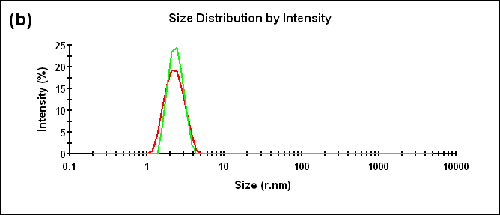
Dual angle DLS measurements were performed on a monodisperse sample of lysozyme. As shown in Table 1 below, the difference between the sizes measured in the backward and forward directions is within the measurement uncertainty (∆Zave = 0.01nm) and the AI is essentially zero (AI = -0.005). The correlograms, as well as the size distributions obtained at the specified detection angles, are compared in Figure 2.
Figure 2a: Correlation functions at 173.0 and 12.8 degrees detection angles.
|
|
Sample | Detection Angle (degrees) | T (°C) | z-Average Radius (nm) |
|---|---|---|---|
Lysozyme | 173.0 | 25 | 2.19 |
12.8 | 25 | 2.18 |
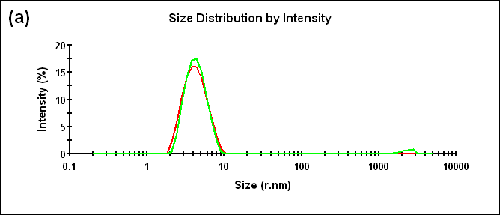
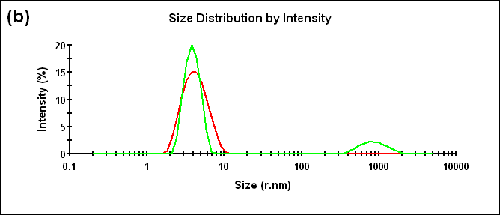
Measurements were also performed on a BSA sample at two different temperatures (25°C and 56°C). The Zave sizes measured in the forward and backward detection angles and the corresponding aggregation indices are shown in Table 2. The size distributions measured at two different angles are shown in Figure 3 (a) and (b) for 25°C and 56°C, respectively.
|
|
Temp (°C) | Detection Angle (degrees) | z-Average Radius (nm) | Aggregation Index |
|---|---|---|---|
25 | 173.0 | 3.93 | 0.05 |
12.8 | 4.14 | ||
56 | 173.0 | 3.86 | 0.1 |
12.8 | 4.26 |
A peak corresponding to the size of aggregates can be observed in the forward scattering angle at the higher temperature. The measured aggregation index increased from 0.05 at 25°C to 0.1 at 56°C.
Dynamic light scattering using a dual angle configuration is shown to enhance detection of aggregates in protein solutions. Moreover, the aggregation index calculated from the Zave sizes measured at two different scattering angles is an early aggregation monitor where higher values indicate a more aggregated protein sample. The effect is enhanced using a forward detection angle of 12.8 degrees, rather than a backscatter angle of 173.0 degrees or the traditional 90 degree detection angle. As such, the dual angle measurement incorporating a forward scattering detector provides an earlier indicator of aggregation onset than would be otherwise possible.