Yu Yuanyuan, Professor Wang Changchun,. Fudan University, Shanghai, P.R.China
Poly(N-isopropylacrylamide) (PNIPAM) is a thermo-sensitive polymer, which undergoes a reversible phase transition from an extended configuration to a condensed structure when the temperature is higher than the lower critical solution temperature (LCST). The thermo-responding property of the PNIPAM is maintained even when PNIPAM acts just as a co-component in the compound. Due to its unique thermo-transition property, PNIPAM has the potential to be applied in chemical and medical areas, such as heterogeneous catalysis and controlled drug release. In this study, the physical properties of size and zeta potential of PNIPAM coated silica particles are studied as a function of environmental temperature and pH using light scattering techniques [1,2].
The PNIPAM coated silica particles were measured using the Zetasizer Nano ZS. This instrument has the capability to perform particle size and zeta potential measurements in a controlled temperature range 0 to 90°C.
The size of the particle was measured by dynamic light scattering at an angle 173°. The hydrodynamic diameter (z-average diameter) was obtained using the Stokes-Einstein equation
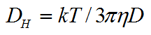
|
The zeta potential was measured by measuring the change of the frequency or phase of the scattered light at a forward angle due to the particles' electrophoretic mobility. From this information we can obtain the mobility of the particle UE, which is related to the zeta potential by the Henry equation:
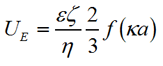
|
Where e is the dielectric constant, ζ is the zeta potential, η is the viscosity, f(κa) is the Henry function, a is the radius of the particle and κ is the reciprocal of the Debye length. In the case of aqueous solutions, where κa >> 1, the Smoluchowski approximation f(κa) = 1.5 is used.
The PNIPAM coated silica particles were kindly provided by the Department of Macromolecule Science, Advanced Material Laboratory, Fudan University, Shanghai. The mono-dispersed silica core was synthesized according to Stöber's method [4] (TEM images shown in figure 1), and PNIPAM was later coated onto the silica by solution polymerization using a crosslink agent. The chemical components used in the synthesis mean that amino and sulphate groups are present in the coating layer. In the final step, the particles were dispersed in water.
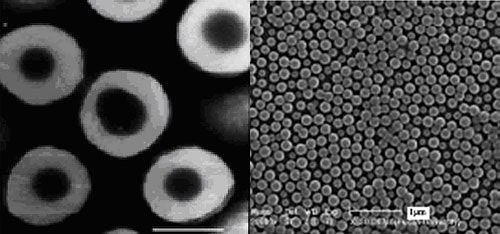
|
The stock sample is a concentrated brown dispersion. For light scattering measurements, the sample was diluted by adding 2 drops of stock dispersion into 20 ml distilled water.
The size and zeta potential were measured as a function of increasing and decreasing temperature across a range 25 to 51°C. The temperature interval used was 1°C per step with an equilibration time of 3 minutes for each measurement. These measurements can be automated in the Zetasizer software which also modifies the viscosity and dielectric constant of water according to the measured temperature.
The pH dependence of the Z-average diameters and zeta potential of the particles were measured across a pH range of 6.5 to 12 using a Zetasizer Nano ZS combined with an MPT-2 autotitrator. 0.25M and 0.025M NaOH solutions were used as titrants for adjusting the pH.
DLS temperature trend measurements were performed across a temperature range of 25 to 51°C. As can be seen in figure 2, in the increasing temperature trend, the size of the particles start to decrease when temperature is higher than 30°C. Over the range of measured temperatures, the diameter of the particles shrink from around 800nm to 300nm. In the case of the decreasing temperature trend, a similar behavior was detected with the exception that the transition temperature was shifted by several degrees to a lower temperature.
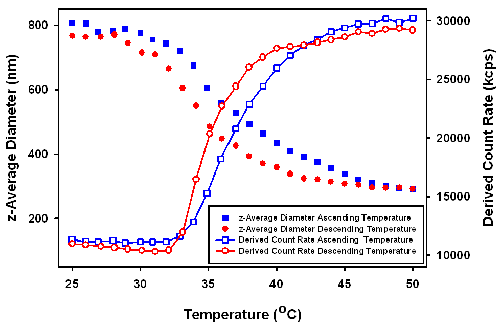
|
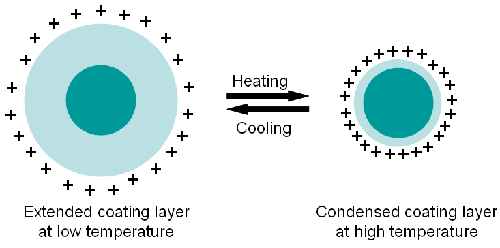
Unlike pure PNIPAM polymers, which have a sharp coil-globular transition around 31 to 33°C [5], the PNIPAM coated silica changed size over a very wide temperature range [1]. Since the size of silica core will not change with temperature, the measured decreasing dimension of the particles must be due to the shrinkage of the PNIPAM layer coating the surface.
The thermo-induced phase transition of the PNIPAM has been interpreted by many researchers. One of the most popular explanations is that above the LCST, the hydrogen bonds between the polymer chains and the aqueous solvent molecules break down causing the PNIPAM to be more hydrophobic. This leads to the collapse of PNIPAM from a coil-like configuration into a condensed structure. Since the breaking of the hydrogen bond needs energy, hysteresis of the phase transition when increasing temperature, is observed.
The derived count rates (in kilo counts per second (kcps)), obtained from the measurements were also plotted in figure 2 as a function of temperature. The derived count rates increases with increasing temperature wheras the decrease in particle size would be expected to result in reduced scattering. The increasing scattering intensity with increasing temperature is proposed to be due to a change in the relative refractive index of the more condensed outer PNIPAM layer.
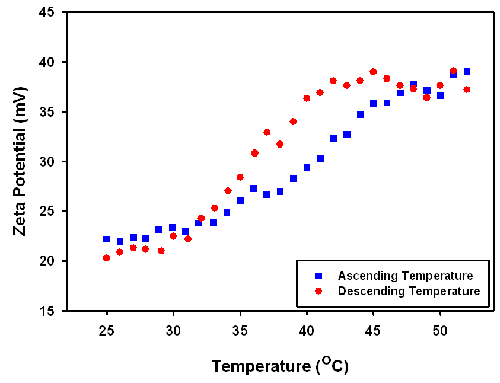
The zeta potential of the particles was also studied as a function of increasing and decreasing temperature. The results are displayed in figure 3. The measured positive zeta potential indicates that the particle carries a positive charge. The results also show that the zeta potential increases with increasing temperature from around 20 mV to 40 mV with the change in the zeta potential being reversible. The variance of the temperature slightly changes the pH and ionic strength. Therefore, the increasing zeta potential values obtained could be due to the shrinkage of the particles leading to a higher charge density on the particle surface (figure 4).
|
|
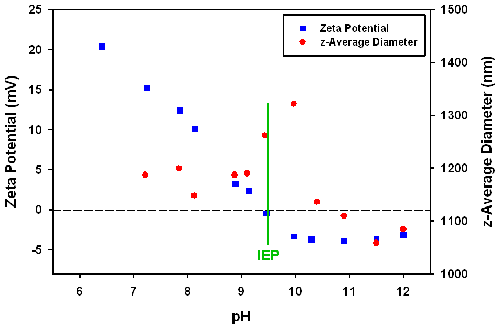
The pH dependence of the zeta potential and size were measured using Zetasizer with an MPT2 autotitrator accessory. The titration started from pH 6.5 and NaOH titrants were added. As shown in figure 5, increasing pH resulted in a decrease in the zeta potential.
|
An isoelectric point was found at pH 9.5, where the zeta potential is zero. It is known that the particles contain both amino and sulphate groups in the coating layer, which provides positive and negative charges, respectively. Adding NaOH to the solution not only decreases the number of the positive protonated amino groups, but also raises the ionic strength. With these two effects, the zeta potential decreases steeply. Below the IEP, the particle size reaches a maximum around the IEP point. This could be due to the formation of aggregates caused by a reduction in electrostatic repulsion.
Size and zeta potential characterization of the PNIPAM coated silica particles as a function of the temperature and pH values were studied using light scattering techniques. The diameter of the particles show reversible changes with changes in temperature. The size of the particles decreased from 800nm to around 300nm over the temperature range from 25 to 51°C. The size of the coated particles also reaches a maximum at pH 10. This tells us that the thickness of the PNIPAM coating layer can be controlled by manipulating these environment parameters.
Changing the temperature increases the zeta potential on the PNIPAM coated particles. This is probably due to the increase in the surface charge density with decreasing size. Both pH and ionic strength influence the zeta potential, therefore they should both be taken into account.
[1] Liusheng Z. et al (2002) Advanced Materials 14, No15, 1090-1092.
[2] Haifeng G. et al (2005) Polymer 46, 1093-1097.
[3] International Standard ISO22412:2008 Particle size analysis, Dynamic Light Scattering (DLS)
[4] Van Blaaderen A, Vrij A. (2002) Langmuir 8:2921-2931.
[5] Wang X., et al, (2002) Macromolecules 31, 2972.
Key Laboratory of Molecular Engineering of Polymers (Ministry of Education), Department of Macromolecular Science and Advanced Materials Laboratory, Fudan University are thanked for their kind provision of the sample and discussion.