Due to growing environmental contamination problems, friendly water-based paints are being increasingly used for various applications. The stability of the paint dispersion is important and is closely related to the quality of the painting products. Traditionally, in order to study the stability of paints produced by different batch and chemical synthesis methods, the paints are placed at room temperature for several months and deposition occurrence and phase separation is studied. In this study, researchers from HLS Paint (Shanghai) Co., Ltd. are trying to identify the stability of epoxy polyester cathodic electrophoretic coating particle dispersions over short time periods using Zetasizer Nano ZS and comparing the results obtained with more traditional methods.
The coating particle dispersions were measured using a Zetasizer Nano ZS, which is capable of both dynamic (particle size) and electrophoretic light scattering (zeta potential) measurements.
Two paint samples, 305# and 803# were received from HLS Paint Co., Ltd synthesized by different chemical polymerization methods (Figure 1). It was reported that paint 803# was stable with good product quality, whereas paint 305# was not stable with deposit found in the paint after a few months.
|
The zeta potential of many samples can be measured directly if their turbidity is low enough. However, when they become too turbid and require dilution, the way in which this dilution is done is absolutely critical in determining the final value measured [1]. Ideally, for these samples, the dilution medium should be identical to that of the original concentrated paints. However, it was not possible to obtain such a dilution medium in this study and therefore, both samples were identically diluted 1 in 40 with distilled water to ensure that the relative zeta potential values obtained were reliable. The particle size and zeta potentials were measured in triplicate at 25°C on a Zetasizer Nano ZS. To stimulate the instability of the sample, the size of the paint particle in the diluted dispersions were also measured at 80°C.
|
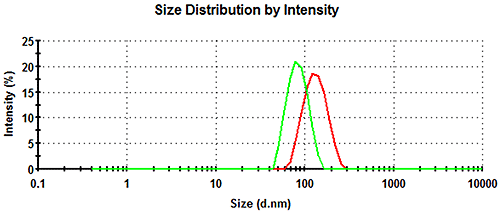
The intensity particle size distributions obtained for the diluted samples 305# and 803# at 25°C are shown in Figure 2 and both the size and zeta potential results obtained are summarized in Table 1. The z-average diameter of paint 305# was 125nm with a polydispersity index (PdI) of 0.058. The z-average diameter of paint 803# was 80.3nm with a PdI of 0.036. The z-average diameter is the intensity-weighted mean diameter and the polydispersity index is a dimensionless measure of the broadness of the size distribution. Both of these parameters are calculated from the cumulants analysis as defined in ISO13321 [2] and ISO22412 [3].
Zeta potential mean values of the two samples were 55.0 and 51.1 mV respectively. Both of the diluted samples show narrow size distributions with high positive zeta potential values. These size and zeta potential results obtained at 25°C cannot provide any information on the stability difference of these two paint samples.
The stability of a dispersion is related to the particle-particle interactions present in the sample and these could be influenced by temperature. The Zetasizer series has built in temperature control allowing for measurements to be made from 0 to 90°C. Therefore, size and zeta potential measurements were performed at a temperature of 80°C.
|
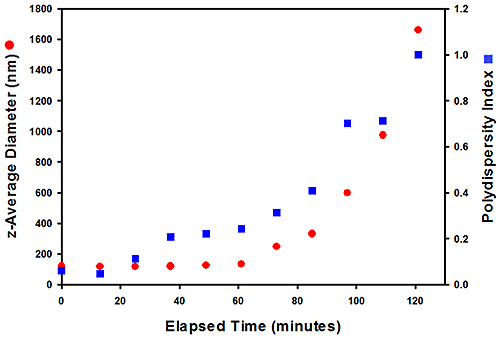
Figure 3 shows the change in the z-average diameter and polydispersity index over time for sample 305# measured at 80°C. The size remains constant for around 40 minutes but then gradually increases and reaches a diameter of 1.8µm after 2 hours. The polydispersity index values also show similar trends. This increase in both size and PdI values is a result of an increasing amount of aggregates being produced at elevated temperature.
|
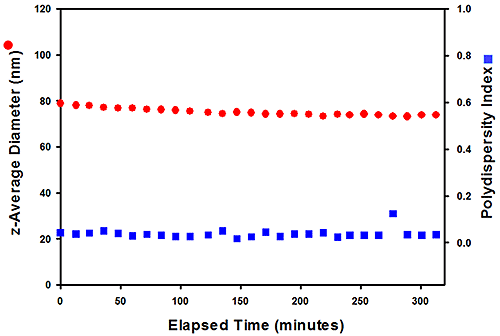
A similar experiment was performed with the 803# paint sample. The results obtained are presented in Figure 4 and show that the sample remains stable over a 320 minute period. For this sample, both the size and PdI values are constant during the measurement with no large aggregation detected.
| z-Average Diameter (nm) | Polydispersity Index (PdI) | Zeta Potential Mean (mV) | |
|---|---|---|---|
| 305# | 124.9 ± 0.4 | 0.058 ± 0.021 | 55.0 ± 0.4 |
| 803# | 80.3 ± 0.2 | 0.036 ± 0.011 | 51.1 ± 1.8 |
The size and zeta potential of two water based paint samples were measured by Zetasizer Nano ZS. At 25°C, the measured size and zeta potential show no indication of any stability difference between the two samples. However, size measurements at 80°C show that the 305# paint sample aggregated after heating for 40 min, while the 803# paint sample remained stable over a 320 minute duration.
This stability difference is in agreement with the user's observations seen on storage of the samples. The use of the Zetasizer Nano ZS allowed identification of the stability of the paint products to be determined on much shorter timescales than traditional tests, greatly increasing the efficiency of QA/QC processes.