Contributed by Dr David Fairhurst, Particle Sciences Inc.
Particle size and zeta potential are fundamental parameters that control stability of suspensions. The present study was conducted to optimize manufacturing process variables (chemical composition, temperature, degree of homogenization, cooling rate, etc) by studying variations that occur in either, or both, the particle size (PS) and zeta potential (ZP) of a commercially available raw material used in skin care product formulation and manufacture.
The raw material in question is an encapsulated retinol system and it is, like many typical cosmetic creams and lotions, a highly complex multi-component formulation. By characterizing the functionality of this dispersed suspension it is possible to gain an understanding of the critical factors that control the process, providing quality assurance for the final product and identifying the surface chemistry that provides the optimal level of product functionality for the final product.
Skin is a unique, continuous external covering that protects us from toxic environmental elements and disease. The skin is also part of the natural resistance of the body; it protects us against invasion by microorganisms. Yet despite its well-known barrier properties, skin itself must be protected.
The aging process of the skin can be divided into two categories; (1) intrinsic aging or chronological changes and (2) extrinsic aging or photo aging, resulting from continuous exposure to ultra-violet radiation and other environmental factors. Both aging processes occur simultaneously, thus complicating any study of skin. It is known, however, that photo aging results in noticeable differences to the skin's character such as, wrinkles, roughness, sallowness, mottled dyspigmentation, and a variety of benign and malignant neoplasms [1]. Skin is in constant danger of oxidative injury because it is continuously exposed to irradiation by visible and ultra violet light. It is well known that membrane damage occurs in the skin lipids because of exposure to UV rays.
Retinol (Vitamin A) has been shown in several studies to have regenerative effects on photo-damaged skin [2]. The most remarkable property of Vitamin A is its stimulating effect on cell proliferation. This results in thickening of the epidermis, increasing formation of collagen and regulation of keratinization in the epidermal cells. The thickening of the epidermis not only improves the barrier function of the skin but, also its appearance and elasticity. Regulation of keratinization decreases the tendency of the skin to dry and flake. Therefore, prolonged topical use has been shown to reduce facial wrinkles. Retinol is also effective in the treatment of acne.
Although retinol has been shown to provide very beneficial effects to the skin, it is a very difficult material to formulate into delivery systems for topical application such as creams and lotions. This is because it suffers extreme instability during almost any formulation process. Retinol degrades through exposure to heat, light, air or common iron oxides and other metal ions and this results in the loss of activity. Retinol is also a very expensive chemical (approximately $2550/kg), thus even a small loss in activity has serious economic implications. Many manufacturers are therefore forced to formulate their skin care products with derivatives of retinol, which do not provide equivalent benefit for the skin. One example is retinyl palmitate, which is more stable, but exhibits significantly lower biological activity. A derivative of Vitamin A, trans Retinoic Acid (Tretinion), is the form most often used in dermatological preparations. However, trans Retinoic Acid has a high irritation potential. This and other side effects restrict its use to prescription formulations.
Particle Sciences Inc (PTI). manufactures, and sells, specialty raw materials to the cosmetics and pharmaceutical industries. One recently developed product is a unique encapsulated form of retinol, designed for controlled release of the active after topical application. Importantly, the encapsulation mitigates the problems of retinol degradation (Figure 1). The proprietary encapsulation process was originally developed for organic sunscreens [3] and has recently been successfully applied to a wide variety of actives from vitamins to insect repellants.

|
Particle size (PS) and zeta potential (ZP) are fundamental parameters that control stability of suspensions [4,5]. The present study was conducted to determine if the manufacturing processing (chemical composition, temperature, degree of homogenization, cooling rate, etc.) resulted in variation(s) of either, or both, the PS and ZP.
Like many cosmetic creams and personal care lotions, the encapsulated retinol system is a highly complex multi-component formulation. However, the basic matrix is essentially a combination of waxes. Accordingly, three compositions were prepared such that the chemistry of the matrix was different (Table 1).
| Formulation
Ingredients | Product A
Ingredient % | Product B
Ingredient % | Product C
Ingredient % |
|---|---|---|---|
| Ceteareth-20 | 15 | 15 | 15 |
| Tocopherol(Vitamin E) | 1 | 1 | 1 |
| Retinol (Vitamin A) | 3 | 3 | 3 |
| Paraffin | 1 | 1 | 1 |
| Water | 53 | 48 | 45 |
| Non-polar Wax | - | 5 | - |
| Polar Ester | - | - | 8 |
The first system, Product A, is a control product. The second system, Product B, contains a higher proportion of a less polar wax (to impact the hydrophobic/hydrophilic balance). A third system, Product C, contains a higher proportion of a polar ester to increase solubilization of the retinol and also to impact the "feel" of the wax (i.e. how it spreads) and "rub-out" (i.e. how it dries on the skin).
It is known, from earlier work on encapsulated sunscreens, that the PSI encapsulation process results in a sub-micron size dispersion, hence a Dynamic Light Scattering (DLS) - based sizing technique was deemed appropriate for the determination of the particle size (PS) and particle size distribution (PSD). In addition, the process utilizes a combination of anionic and non-ionic surfactants; hence the choice of a Malvern Zetasizer to determine the resultant zeta potential (ZP) of the particles.
Measurements were conducted on diluted samples of the production concentrates using the Malvern Zetasizer Nano ZS90 instrument. The data presented is the average of six runs for each sample.
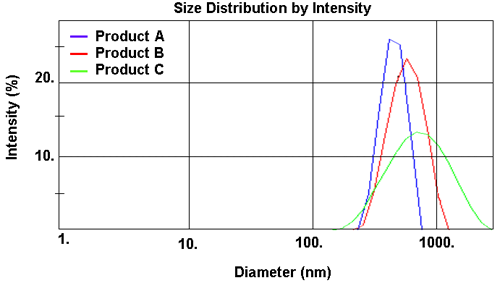
The results obtained are summarized in Table 2. Each product has an average particle size around 600nm with a fairly broad distribution, but there are observable differences. A small, but definite, increase in both size and polydispersity occurs from Product A through to Product C (clearly seen in Figure 2). This is reflected in a measured change (increase) in viscosity from 25cP to 60cP (Table 3). From a commercial perspective, the requirement for shelf storage of these materials is in excess of one year. Thus, with a mean particle size significantly less than 1µm and with the solid matrix having a density virtually equal to that of water, each system should be stable to sedimentation and/or creaming, with Product C being the least stable. This has been subsequently confirmed by accelerated aging of the suspensions at 45°C.
|
| Size Measurement | Product A | Product B | Product C |
|---|---|---|---|
| z-Average Diameter (nm) | 587 | 660 | 795 |
| Polydispersity Index | 0.216 | 0.352 | 0.531 |
| Sample | Viscosity (cP) |
|---|---|
| Product A | 25 |
| Product B | 36 |
| Product C | 60 |
It is also important that the PS be kept smaller than 1µm in order to maximize surface area coverage (for efficient delivery of active) and also to minimize rub-out time. The change in composition of the matrix obviously affects the final PS of the dispersion, which is not totally unexpected since the substitute components each have different melting points, hardness, chemistry etc. This aspect is currently under further study since it directly impacts the economics of the production process.
Zeta Potential
Figure 3 shows the variation in Zeta Potential with pH for the three production samples. The measurements were made on the Malvern Zetasizer with diluted samples.

|
It is clear that Product B exhibits a different profile from both Product A and Product C. Product B has the matrix that contains the non-polar wax. It is known that waxes have, what is termed, an "acid number". This is a function of the number of acid sites arising from the presence of any free fatty acid in the wax; the higher the free fatty acid content, the larger the acid number.
Since the isoelectic point (i.e.p.) for Product B (at pH2.0) is shifted to a lower pH compared with Products A and C (pH 2.4), it suggests that this wax component is predominantly at the surface of the encapsulation matrix. This is supported by the observation that the individual ZP values are all smaller than those of the control material.
This is an important finding because the retinol dispersions are used to formulate creams and lotions that, typically, need to be "pH neutral". Understanding the surface chemistry allows the formulator to determine what the potential reactivity might be.
Product C, containing the polar ester, does not show any difference from the control, Product A. This implies that the ester is solubilized within the wax matrix and is not present at the surface. This finding is not unexpected since the primary reason for adding the ester was to improve the solubilization of the tocopherol and retinol. However, it is useful to be able to confirm the hypothesis and critical to understanding the interfacial properties of the product.
The R&D Department at PSI is now using the Malvern Zetasizer Nano to study the impact of surface chemistry on formulation issues such as understanding of the surface and interfacial properties as well as long-term product stability.
1. R. Marks, "Sun-damaged Skin", Martin Dunitz Publishers, London (1992)
2. C.C.Zouboulis, IFSCC, 3 9 (2000)
3. D.Fairhurst and M.Mitchnick, Cosmetics and Toiletries, 110 47 (1995)
4. G.D.Parfitt (Ed), "Dispersions of Powders in Liquids", Applied Science Publishers, New York (1981)
5. D.Fairhurst, Tire Technology International, 151 (1996)