How the surface zeta potential cell works and how it can be used to determine the surface charge of for example PTFE in 1mM NaCl at pH9.2, as observed by 300nm tracer particles DTS1230. The surface zeta obtained was -85mV.
In aqueous systems, the majority of particle and wall surfaces will acquire a charge of some kind. Charged regions or the disassociation of charged groups on the material surface will lead to the attraction of buffer salts to the surface. The closest salts will be strongly attracted forming the Stern layer at the surface of the material under study. Further from the surface, buffer salts will be attracted to the material but will not be closely bound and will be exchanged with the rest of the salts in the medium. This exchangeable layer is the diffuse layer. Zeta potential is defined as the charge at a notional boundary within the diffuse layer and is called the slipping plane. Zeta potential is therefore highly dependent on both the sample and the medium in which it is immersed.
Zeta potential is commonly used as an indicator of stability of particle suspensions and colloidal samples. In principle, a high zeta potential (either positive or negative) should provide sufficient repulsive energy to the particles to maintain particle distance and protect sample stability by preventing flocculation and aggregation.
Zeta potential is often measured using the technique of laser Doppler electrophoresis. Briefly, the frequency change in a laser beam is measured after scattering by the sample particles undergoing electrophoresis. The frequency shift is related to the particle velocity, which in turn is related to the strength of the electric field and the zeta potential of the sample.
Although zeta potential measurements are commonly used to measure particles in suspension, it is also true that the surfaces of any container or non-suspended material will also acquire a charge and it may be desirable to measure the zeta potential of these surfaces. Since, by its nature, a wall or solid surface cannot be made to undergo electrophoresis, a modification of the laser Doppler electrophoresis technique is required to measure this zeta potential.
Typically, a technique such as streaming potential is used to measure surface potential. However, this requires specialist equipment dedicated to the measurement.
This application note describes the measurement of surface zeta potential using the zeta potential planar cell along with tracer particles. By measuring the electrophoretic mobility of the particles at varying distances from the planar surface, the magnitude of the particle electrophoresis and the electro-osmosis generated by the wall zeta potential can be used to calculate the zeta potential at the wall surface.
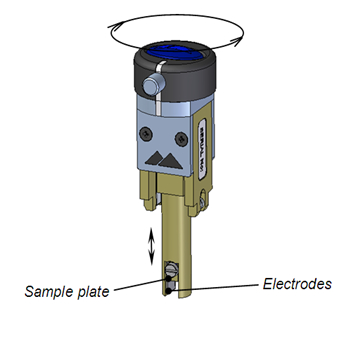
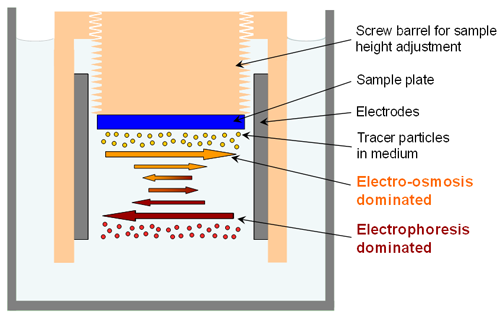
Figure 1 shows a diagram and photo of the surface zeta potential measurement cell with a height adjustable sample holder situated between two electrodes. Figure 2 shows a close-up schematic of the measurement. The cell is in a dip cell format with a rotating top attached to a screw thread allowing the sample height, and thus the displacement, to be altered. The sample is attached to the cell and submersed in a medium containing tracer particles. The application of an electric field via the electrodes will initiate electrophoresis of the particles. Close to the sample surface, electro-osmosis will also be established. The mobility of the particles in the system will be a balance between these two forces. The closer the tracer particle to the sample surface the greater the significance of the electro-osmosis on the particle mobility. As the particle distance from the surface increases, the effect of the electro-osmosis will decrease to the point at which particle movement is entirely dependent on electrophoresis.
|

|
|
Particle electrophoretic mobility is measured using the well-established M3-PALS technology in the Zetasizer Nano.
It is clear that the measured electrophoretic mobility will therefore vary as a function of distance from the sample surface. By plotting the reported mobility, or zeta-potential, as a function of displacement from the surface, the relationship can be extrapolated back to the intercept, or zero displacement.
Since the measured zeta potentials always include a component of the electrophoretic motion, the measured intercept will necessarily include this component which must be accounted for. In fact, the wall potential is defined by equation (1):
ξwall = -intercept + ξparticle(1)
The following is an example of a surface zeta potential measurement using the surface zeta potential cell. The zeta potential of a PTFE surface was measured in 1mM NaCl at pH 9.2 using 300 nm latex particles as the tracer particles (DTS1230).
Measurements were made at 125, 250, 375 and 500μm from the sample surface. The final measurement at 500μm is an electrophoresis-only measurement to determine the zeta potential of the tracer particles. This can then be fed into equation (1) to determine the zeta potential of the PTFE surface from the calculated intercept of the displacement plot.
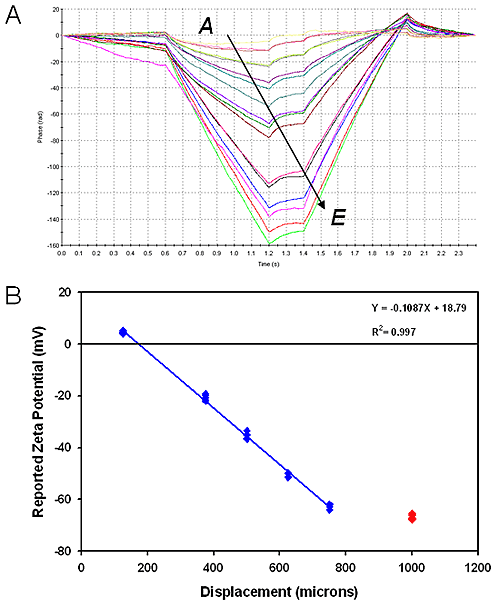
Figure 3A shows phase plots for the measurements with the magnitude of the change in scattered light frequency increasing with the distance of the measurement from the surface.
|
Figure 3B shows a displacement plot of reported tracer particle zeta potential as a function of displacement from the sample surface. From the graph, the intercept is calculated to be approximately 19 mV and from equation (1), the surface zeta potential of PTFE is calculated to be -85.5 ± 1.2 mV.
Any surface in an aqueous environment will have a zeta potential and this potential induces electro-osmosis in the presence of an applied electric field. Particles in the suspension will themselves have a charge and move under electrophoresis upon application of the electric field. Using suspended particles as tracers, the magnitude of the surface zeta potential can be measured using the surface zeta potential cell by measuring the particle mobility at multiple distances away from the sample surface.
In this study, a sample of PTFE in 1mM NaCl pH 9.2 has been shown to have a surface zeta potential of -85.5 mV.